Lesson: Mass and Ksp
advertisement

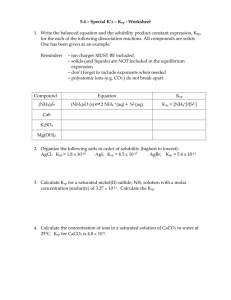
Lesson #4: Mass and Ksp When mass is given, it is necessary to convert mass to moles in order to calculate the concentration of the resulting ions. 1. 2.50g of calcium carbonate just barely dissolves in 50.0mL of water. Calculate Ksp. Step #1: convert mass to moles. Step #2: Determine the concentration of solid that has dissolved. Step #3: Write a dissociation equation. Step #4: Write the solubility product expression. Step #5: Use a mole ratio to determine the concentration of ions in solution. Step #6: Sub-in and solve for Ksp. (Does this match the Ksp value in the data book?) Work from Ksp back to Mass 1. Calculate the maximum mass of Zn(OH)2 that will dissolve in 2.0L of water if the Ksp = 7.9 x 10-8 (Does this Ksp match the one in the data book?) Step #1: Write the dissociation equation. Step #2: Write the solubility product expression. Step #3: Determine the mole ratio and apply appropriate “Let statements” Step #4: Plug in knows/unknowns into Ksp expression and solve. Step #5: Use ratio to determine the concentration (M) of solid dissolved. Step #6: Convert moles to mass. Step #7: Adjust the mass for the given volume. (in other words: Do you have 1.0L of solution?)