Exam 2 Review WS
advertisement

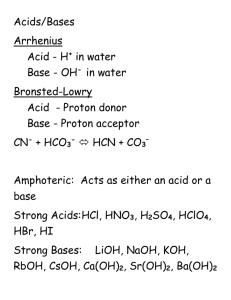
SI Chem 178 Exam 2 Review Leader: Emily Date: March 11/12, 2013 Exam Covers: Ch 16.6-16.11, Ch 17.1-17.6 Concept Review: 16.6/16.7 Weak Acids and Bases Write out the chemical equation and Ka equation for dissociation of an acid and a separate one for bases (Kb.) Strong acids have ___________ conjugate bases. Weak acids have __________ conjugate bases. T / F A large Ka value means the acid is weak T / F A large pKa value means the acid is weak T / F Small % ionization means the acid is weak. Write out the % ionization equation: T / F The first proton in a polyprotic species is harder to remove than the second (What do we know about the acidity of each of these H’s) When can we treat them as monoprotic? Given pH values in weak acid problems tell you about initial OR equilibrium conditions? How do you get to concentration from pH? Which concentration(s) does this tell you? Given a Ka value and initial concentration of a weak acid, what can we find? What assumptions might we make? How do we check their validity? What are some helpful things to write down when solving weak acid/base problems? (Discuss with a partner how you approach them!) 16.8 Relating Ka, Kb: What are the equations that relate Kw, Ka, and Kb and pKw, pKb, and pKa? Can these relate two DIFFERENT acid species? 16.9 Salt Solutions Cr3+, Al3+, and Fe3+ are metals that make solutions ACIDIC or BASIC. How? Do cations like Ca2+ and Na+ have this same effect? Conjugates of strong species affect pH…. YES or NO The conjugate of a weak acid would make a solution basic… YES or NO NX4+ is the conjugate ______ of _______ and makes a solution ________ 16.10 Acid Strength H-X acid strength is affected by ___________ Oxyacid strength is affected by ______________ and ______________ How to remember this? Consider HF and HI… Consider HClO and HClO3 or HClO4… In carboxylic acids, _________ helps contribute to the stability of their ______ _______. 16.11 Lewis Acids and Bases Define: Lewis acid:__________________________________________ Lewis Base:________________________________ What is a common type of LB? 17.1 The Common Ion Effect Ionization INCREASES or DECREASES if a common ion is in solution? Does this follow Le Chatelier’s principles? Where do we place the concentration of the salt/strong electrolyte in our ICE table? I/E? What contributes to pH in these cases? 17.2 Buffers T/ F Large swings in pH are observed with buffers upon the addition of acidic/basic species Buffers are made with STRONG or WEAK acid/base species? What is the HH equation? In a CH3COOH-CH3COO- buffer, what happens when HCl is added? What about NaOH? Therefore: SA reacts with ________ component of buffer and SB reacts with _________. Which has more buffer capacity: 1L of 2M benzoic acid and 2M sodium benzoate, or a 1L solution of 0.2M benzoic acid and 0.2M sodium benzoate? What can you say about their pH values? What sort of tables do we use for buffer problems? 17.3 Acid-Base Titrations 1. Draw a curve of an acid being titrated by a base. 2. Write the calculation steps at each of the 4 points that would be required for a STRONG acid and what would have to be done for a WEAK acid. What is the pH at the equivalence point of an SA/SB titration? Is the pH acidic, basic, or neutral at the equivalence point of a weak acid titrated with a strong base? (Circle one.) What about a weak base titrated with a strong acid? Indicators should have a pH that falls near the titration’s_______________________ 17.4 Solubility Equilibria How do we convert from mass solubility (g/L) of a compound to Ksp? (Diagram pg 724) 17.5 Solubility Factors Write “increase” or “decrease” for the following manipulations and their effect on solubility. ________________ Putting a common ion in solution _________________ Acidifying the a solution of a salt with a basic ion _________________ Adding base to transition metal (ex: Ag+) salt solutions (complex ions concept) 17.6 Precipitation/Separation of Ions Write what occurs in each case: Q > Ksp _____________________________________________________ Q < Ksp ________________________________________________________ Q=Ksp _________________________________________________ Species with HIGHER/LOWER Ksp precipitate out first (at equal concentrations.) Problems: 16.61 Saccharin (HNC7H4SO3) has a pKa of 2.32; what is the pH of a solution of 0.10M saccharin? What is its Kb? pKb? Acidic, Basic or Neutral? Fe(NO3)3 CH3NH3 Cl NH4NO3 KBr NaF AlCl3 (CH3COO)2Ba NaHC2O4 KCN 16.98 ID the Lewis Acid and Lewis Base in each of the following reactions: 1. HNO2(aq) + OH- (aq) NO2- (aq) + H2O (l) 2. FeBr3(s) + Br- (aq) FeBr4- (aq) LA_________ LB_________ LA________ LB ____________ 3. Zn2+(aq) + 4NH3 (aq) Zn(NH3)4 2+ (aq) LA ________ LB __________ 4. SO2 (g) + H2O (l) H2SO3 (aq) LA____________ LB____________ Ch 17 p. 706 Common Ion Effect Problem: Calculate the pH of a solution that is 0.2M HF and 0.1M HCl. Ka of HF =6.8x10^-4 17.21 Calculate the pH of a buffer that is 0.12M in lactic acid and 0.11M sodium lactate. What would the pH be if we combined 85mL of 0.13M lactic acid with 95 mL of 0.15M sodium lactate? (Ka of lactic acid is 1.4x10^-4) Calculating pH in a weak acid/strong base titration: Refer back to titration curve/conceptual question! What is the definition of equivalence point? 1. What is the pH of 50mL 0.100M acetic acid (CH3COOH, Ka=1.8x10^-5) 2. What is the pH after 45mL 0.100 NaOH has been added? 3. What is the pH after 50mL of 0.100M NaOH has been added? What point is this? What do we expect the pH to be? 4. What is the pH after 75mL NaOH has been added? 17.59. Is Mn(OH)2 most soluble at pH 7, 9.8, or 11.8 (circle one) 17.54 A flask sitting at 25DegC holds 1L of water with 0.54g PbI2 in it. What is its Ksp? 17.72 A solution contains 0.01M Sr2+ and 0.01M Ba2+, both of these ions complex with SO4-2 and can come out of solution. Which is expected to come out first? What is the concentration required to start this precipitation of the first compound? What about the second? Ksp values: BaSO4 = 1.1 X 10^-10 SrSO4 = 3.2 x 10^-7