The Solubility Product: A Special Equilibrium Case
advertisement

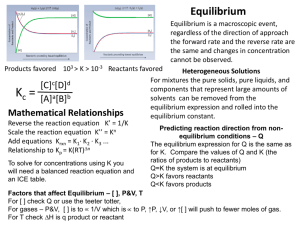
The Solubility Product: A Special Equilibrium Case Information Not all ionic compounds dissolve readily in water. For example, when a lump of Mg(OH)2 solid is placed in a beaker containing 1.00 liters of water at room temperature, only 1.65 x 10-4 moles of the solid dissolve. Once equilibrium is established between the solid material and the aqueous species, the solution is said to be saturated. The solubility of Mg(OH)2 is 9.63 x 10-3 grams/liter. The equilibrium constant, K, for dissolving an ionic solid in water is given a special name (It’s one of the “special K’s”). It is called the solubility product, Ksp. Model : Mg(OH)2 is insoluble in water Critical Thinking Questions 1. Why are six hydroxide ions shown in solution in the model whereas there are only three magnesium ions shown? 2. How was the value for the solubility of Mg(OH)2, 9.63 x 10-3 grams/liter, obtained from the fact that 1.65 x 10-4 moles of the solid dissolves in one liter of water? Show the calculation. 3. Calculate the Mg2+ concentration and OH- concentration in moles/liter. 4. Write the equilibrium expression for the Ksp of Mg(OH)2 and use it to determine the value of the Ksp for Mg(OH)2. Note: Solids do not appear in the expression!!!! 5. Consider a solution formed by combining 500.0 ml of a 0.12 M NaOH solution with 500.0 ml of 0.10 M Mg(NO3)2 solution in a beaker. a). How many moles of Mg2+ (aq) are present (assuming no reaction occurs)? b). How many moles of OH- (aq) are present (assuming no reaction occurs)? c). What is the volume of the final solution? d). At this point (assuming no reaction occurs) what is [Mg2+]? e). At this point (assuming no reaction occurs) what is [OH-]? f). Write the expression for the reaction quotient, Q, for this reaction. Mg(OH)2 (s) Mg2+ (aq) + 2OH- (aq) Calculate the value of Q for the mixture as described thus far. g). Upon mixing, is the system at equilibrium? If not, in what direction will it shift? h). Does a precipitate form? Exercises: 1. A saturated solution of Lithium Carbonate is obtained after 0.0742 moles of the solid have been dissolved to make a 1.00 L solution. Calculate the value of Ksp for lithium carbonate. 2. What mass of MgF2 will dissolve in 125 ml of water if Ksp = 6.5 x 10-9.