Galvanic Cell Concept
advertisement
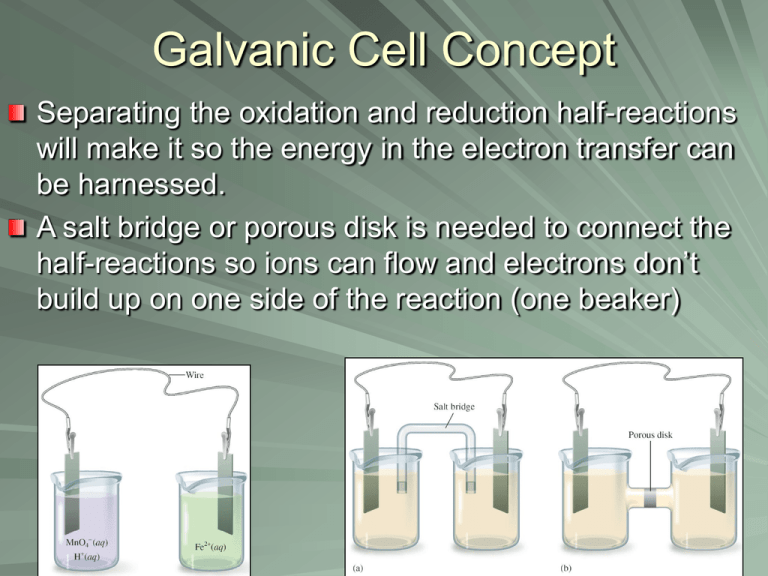
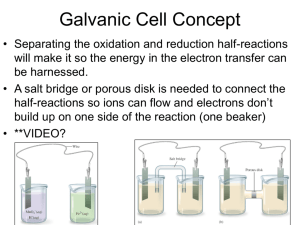
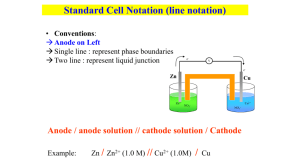
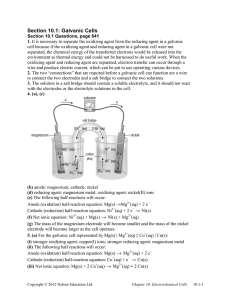
Galvanic Cell Concept Separating the oxidation and reduction half-reactions will make it so the energy in the electron transfer can be harnessed. A salt bridge or porous disk is needed to connect the half-reactions so ions can flow and electrons don’t build up on one side of the reaction (one beaker) Galvanic Cell Definition Device which chemical energy is changed to electrical energy. Oxidation occurs at the ANODE Reduction occurs at the CATHODE (cat gets fat = cathode gains electrons a.k.a. reduction) – An ox and a red cat (anode/oxidation, reduction/cathode) Electrodes If there is an element (not ion) in either half-reaction, it is what that particular electrode is made of – ***comes up later! When all reactants/products are in solution (aq) Pt or graphite can be used as the electrode. Galvanic Cell Picture (Parts) Cell Potential/Electromotive Force (EMF) Represented by E°cell Unit = volt (V) = 1 joule/coulomb Measured with a voltmeter (not completely accurate b/c of heat). A potentiometer is used instead where the maximum cell potential can be measured. Standard Reduction Potentials If we can find the potential for each halfreaction (Table 18.1 pg. 829), we can determine the cell potential (E°cell) Half-reaction manipulations (DO NOT MANIPULATE VOLTAGE): – One must be reversed (oxidation)…can reverse E so you have -E = -voltage… – Electrons lost must = electrons gained, so multiplication of reaction may be needed (DO NOT MULTIPLY VOLTAGE BY THIS NUMBER!) EQUATION (MEMORIZE) E°cell = E°(cathode) - E°(anode) Table 18.1 Better oxidizing agents: easily reduced, LEFT side rxn. = largest, most positive standard reduction potential Better reducing agents: easily oxidized, RIGHT side = most negative standard reduction potential (aka most positive standard oxidizing potential) Example: Which is best reducing agent Cu+, F-, H-, H2O, I2, K (find in reactants) Answer: K (-2.92) > H- (-2.23) > Cu+ (0.16) > I2 (1.20) > H2O (1.23) > F- (2.92) Galvanic Cell Example Calculate the emf values (E°cell) for the following Mg(s) + 2H+(aq) -> Mg2+(aq) +H2(g) Answer: E°cell = +2.37V In Galvanic Cells… When E°cell is positive, the reaction will run spontaneously. (last slide) If negative, it will run in the opposite direction (will NOT run spontaneously as written). Cell Potential, Electrical Work, and Free Energy Spontaneous IF: – Positive cell potential – Negative Gibbs Free Energy (we will learn more about Gibbs Free Energy in a later chapter) Line Notation Not required for AP Exam A double vertical line separates the anode on left and cathode on right – Represents a salt bridge or porous disk A single vertical line separates different phases Ex: anode Cd -> Cd2+ + 2ecathode Hg2+ + 2e- -> Hg Line notation: Cd(s) I Cd2+(aq) II Hg2+(aq) I Hg Galvanic Cell: Complete Description In half-reaction descriptions…FOUR items needed: 1. The cell potential - positive when E°cell = E°(cathode) - E°(anode) and the balanced cell reaction 2. The direction of electron flow, obtained by inspecting the half-reactions and using the direction that gives a positive E°cell. 3. Designation of the anode and cathode. 4. The nature of each electrode and the ions present in each compartment. A chemically inert conductor is required if none of the substances participating in the half-reaction is a conducting solid. Example: Complete Description Describe a galvanic cell based on the two half-reactions below. Cu2+ + 2e- -> Cu E° = 0.34 V Cr2O72- + 14H+ + 6e- -> 2Cr3+ + 7H2O E° = 1.33V 1. Balanced cell rxn: 3Cu(s) + Cr2O72-(aq) + 14H+(aq) -> 3Cu2+(aq) + 2Cr3+(aq) + 7H2O(l) E°cell = 0.99V (needs to be positive) 2. 1.33 (cathode) -0.34 (anode) means Cu needs to be reversed. Cu will be giving off e- which will travel from Cu (anode) to cathode (platinum electrode). Continued… 3. Anode (copper metal electrode), cathode (platinum electrode) 4. The copper metal electrode (anode) will be in the Cu/Cu2+ compartment while the platinum electrode (cathode) will be in the Cr2O72-/Cr3+ compartment Changing Concentration In chemical reactions… Standard conditions = 1M for all When reactants are >1M, it will increase product concentration and will increase the cell potential When products are <1M, it will decrease the product concentration (oppose forward reaction), decreases cell potential Mg(s) + 2H+(aq) -> Mg2+(aq) +H2(g) Concentration Cell A galvanic cell that has the same component on each side but at different concentrations Causes a cell potential Voltages are typically small Ex: a cell has on its left side a 0.20 M Cu2+ solution and a 0.050 M Cu2+ solution on the right side