Zumdahl*s Chapter 17

GALVANIC CELL
SAHAR ADHAM
LECTUERE OF PHYSICAL CHEMISTRY
Comparison of Electrochemical Cells
galvanic electrolytic produces electrical current anode (-) cathode (+) two electrodes conductive medium salt bridge vessel
E ° cell
> 0.
need power source anode (+) cathode (-)
E ° cell
< 0.
ELECTRON TRANSFER REACTIONS
Electron transfer reactions are oxidation-reduction or redox reactions.
Results in the generation of an electric current (electricity) or be caused by imposing an electric current.
Therefore, this field of chemistry is often called
ELECTROCHEMISTRY.
YOU CAN’T HAVE ONE… WITHOUT THE OTHER!
oxidation: loss of electrons
reduction: gain of electrons
LEO the lion says GER !
GER!
WHY STUDY ELECTROCHEMISTRY?
Batteries
Corrosion
Industrial production of chemicals such as Cl
2 and Al
, NaOH, F
2
Biological redox reactions
The heme group
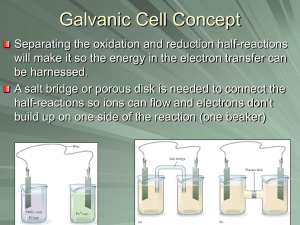
GALVANIC CELLS
One ½ cell rxn. occurs in each compartment.
Zn Zn 2+ + 2e – in the anode.
Cu 2+ + 2e – Cu in cathode.
But not without a connection.
Zn
SO
4
2 –
Zn 2+
SO
4
2 –
Cu 2+
Cu
Zn + Cu 2+
Zn 2+ + Cu
ION (“SALT”) BRIDGE
But even with a connection of the electrodes, no current flows.
We need to allow neutrality in the solutions with a salt bridge to shift counterions.
Zn
2e
–
SO
4
2 –
Zn 2+
SO
4
2 –
Cu 2+
2e
–
Cu
Zn + Cu 2+
Zn 2+ + Cu
Cell Potential
•Cell Potential or Electromotive
Force (emf): The “pull” or driving force on the electrons.
STANDARD REDUCTION POTENTIALS, E °
The voltage generated by the Zn/Cu galvanic cell is
+1.1V under standard conditions .
Standard conditions are:
T = 25°C and P = 1 bar for gases.
Solids and liquids are pure.
Solutions are 1 M in all species.
E ° cell is sum of ½ cell E ° values.
CELL POTENTIALS AND REDUCTION POTENTIALS
E °cell = E °reduced - E°oxidized
E °cell = E °cathode - E°anode
½ CELL REDUCTION POTENTIALS
All ½ cells are catalogued as reduction reactions & assigned reduction potentials, E °.
The lower reduction potential ½ rxn is reversed to become the oxidation. E ° oxidation
= – E ° reduction
That makes spontaneous E ° cell
> 0.
But E ° red can’t be found w/o E ° ox
!
ORIGIN FOR REDUCTION POTENTIALS
We need a standard electrode to make measurements against!
The Standard Hydrogen Electrode (SHE)
2H + ( aq ) + 2e – H
2
(1 bar) E °
0 V
1 bar H
2
E ° cell
E °
SHE flows over a Pt electrode, and the full is assigned to the other electrode.
= 0 V.
E.g., standard calomel electrode:
Hg
2
Cl
2
( s ) + 2e – 2 Hg( l ) + Cl – E °
SCE
= +0.27V
a more physically convenient reference.
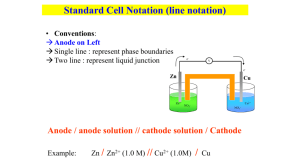
GALVANIC LINE NOTATION
Shorthand for a complete redox cell is of the form:
Anode | anodic soln. || cathodic soln. | Cathode
So making a cell of Cu corrosion,
Cu | Cu 2+ || NO
3
– , NO( g ), H + |Pt
where all ions should be suffixed ( aq ) and both metals should have ( s ).
TYPES OF GALVANIC CELLS
Primary Battery : can not be recharged e.g.
Mercury Battery
Secondary Battery : rechargeable (storage batteries) e.g.
Ni-Cad Battery
Fuel Cell : reactants supplied from an external source e.g.
H2/O2 fuel cells.
MERCURY BATTERY
Anode:
Zn is reducing agent under basic conditions
Cathode
:
HgO + H
2
O + 2e- ---> Hg + 2 OH can not be recharged
NI-CAD BATTERY
Anode (-)
Cd + 2 OH ---> Cd(OH)
2
Cathode (+)
+ 2e-
NiO(OH) + H
2
O + e- ---> Ni(OH)
2 rechargeable
+ OH -
WHY RECHARGEABLE?.
It is because the products of the reaction are solids that the Ni-Cd battery can be recharged
The solid hydroxides are sticky, and remain in place.
If current is applied, the reaction can be driven backwards
!
HOW TO CHARGE?
When you charge a battery, you are forcing the electrons backwards
(from the + to the -). To do this, you will need a higher voltage backwards than forwards
.
WHY NOT RECHARGEABLE ?
But in mercury battery the ZnO is not sticky, and doesn’t remain attached to the electrode. This battery is not rechargeable
H
2
AS A FUEL
Cars can use electricity generated by H
2
/O
2 fuel cells.
H
2 carried in tanks or generated from hydrocarbons
Fuel Cells
Galvanic cells for which the reactants are continuously supplied.
anode:
2H
2
+ 4OH
4H
2
O + 4e
cathode :
4e
+ O
2
+ 2H
2
O
4OH
2H
2
( g ) + O
2
( g )
2H
2
O( l )
17-44
Mercury batteries take advantage of the high density of Hg to be quite small: used in watches, hearing aids, calculators, etc.
Lithium-iodine batteries are particularly small and lightweight, but also very long-lived
Often used in pacemakers, where they can last for 10 years
THE END
V1.06
QUESTION 1
For a galvanic cell, the electrode at which reduction occurs is called the:
A: Anode B: Cathode
ANSWER
For a galvanic cell, the electrode at which reduction occurs is called the:
B: Cathode
QUESTION 2
For a galvanic cell, the electrode with negative polarity is called the:
A: Anode B: Cathode
ANSWER
For a galvanic cell, the electrode with negative polarity is called the:
A: Anode
32
QUESTION 3
Which of the following statements is incorrect a.
b.
In a galvanic cell, reduction occurs at the anode.
The cathode is labeled "+" in a voltaic cell.
c.
d.
Oxidation occurs at the anode in a voltaic cell.
Electrons flow from the anode to the cathode in all electrochemical cells.
ANSWER a.
In a galvanic cell, reduction occurs at the anode.
34
QUESTION 4
Consider the following notation for an electrochemical cell
Zn|Zn 2+ (1M)||Fe 3+ (1M), Fe 2+ (1M)|Pt
What is the balanced equation for the cell reaction?
a.
Zn(s) + 2Fe 3+ (aq) → 2Fe 2+ (aq) + Zn 2+ (aq) b.
Zn 2+ (aq) + 2Fe 2+ (aq) → Zn(s) + 2Fe 3+ (aq) c.
Zn(s) + 2Fe 2+ (aq) → 2Fe 3+ (aq) + Zn 2+ (aq) d.
Zn(s) + Fe 3+ (aq) → Fe 2+ (aq) + Zn 2+ (aq) e.
Zn(s) + Fe 2+ (aq) → Fe(s) + Zn 2+ (aq)
ANSWER
Zn(s) + 2Fe 3+ (aq ) → 2Fe 2+ (aq) + Zn 2+ (aq)
QUESTION 5
What is the oxidation state of nitrogen in
HNO
3
?
A: +3 B: +4
C: +5 D: -5
ANSWER
What is the oxidation state of nitrogen in
HNO
3
?
C: +5
38
QUESTION 6
Consider the following electrode potentials:
Mg 2+ + 2e
–
Mg E ° = –2.37 V
V 2+
Cu 2+
+ 2e
+ e
–
–
V E ° = –1.18 V
Cu + E ° = 0.15 V
Which one of the reactions below will proceed spontaneously from left to right?
a. Mg 2+ + V
V 2+ + Mg b. Mg 2+ + 2Cu +
2Cu 2+ + Mg c. V 2+ + 2Cu +
V +2 + Cu 2+ d. V + 2Cu 2+
V 2+ + 2Cu + e. none of these
ANSWER
d. V + 2Cu 2+
V 2+ + 2Cu +
QUESTION 7
What is the oxidative state of iodine in IO
3
?
A: +7 B: +6
C: +5 D: +4
ANSWER
What is the oxidative state of iodine in IO
3
?
C: +5