Standard Cell Notation (line notation)
advertisement

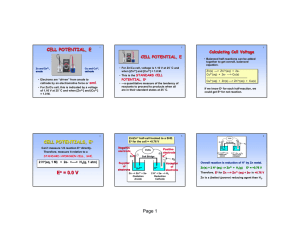
Standard Cell Notation (line notation) • Conventions: Anode on Left Single line : represent phase boundaries Two line : represent liquid junction e- V e- Zn Zn2+ Cu Cu2+ NO3- NO3 - Anode / anode solution // cathode solution / Cathode Example: Zn / Zn2+ (1.0 M) // Cu2+ (1.0M) / Cu Line Notation • solidAqueousAqueoussolid • • • • Anode on the leftCathode on the right Single line different phases. Double line porous disk or salt bridge. If all the substances on one side are aqueous, a platinum electrode is indicated. For the last reaction Cu(s)Cu+2(aq)Fe+2(aq),Fe+3(aq)Pt(s) Cu2+ Fe+2 17_360 e– e– e– e– Porous disk Oxidation e– (a) Reducing agent Anode Reduction Oxidizing agent (b) e– Cathode Copyright McGraw-Hill 2009 7 Practice: In a galvanic cell, the electrode that acts as a source of electrons to the solution is called the __________; the chemical change that occurs at this electrode is called________. a. cathode, oxidation b. anode, reduction c. anode, oxidation d. cathode, reduction Practice Under standard conditions, which of the following is the net reaction that occurs in the cell? Cd|Cd2+ || Cu2+|Cu a. Cu2+ + Cd → Cu + Cd2+ b. Cu + Cd → Cu2+ + Cd2+ c. Cu2+ + Cd2+ → Cu + Cd d. Cu + Cd 2+ → Cd + Cu2+ Galvanic Cell • • 1. 2. 3. 4. The reaction always runs spontaneously in the direction that produced a positive cell potential. Four things for a complete description. Cell Potential Direction of flow Designation of anode and cathode Nature of all the components- electrodes and ions Oxidation Numbers on the Periodic Table Cell Potential • Cell Potential / Electromotive Force (EMF): • The “pull” or driving force on electrons • Measured voltage (potential difference) • The total cell potential is the sum of the potential at each electrode. 17_363 e– e– Zn(s) e– Ecell = +1.10 V + Zn 2 – SO 4 2 1.0 M Zn 2 solution Anode e– + Cu 2 – SO 4 2 + + 1.0 M Cu 2 solution Cathode Cu(s) Cell Potential, E0cell 0 E cell cell potential under standard conditions elements in standard states (298 K) solutions: 1M gases: 1 atm Practice • Completely describe the galvanic cell based on the following half-reactions under standard conditions. • MnO4- + 8 H+ +5e- Mn+2 + 4H2O Eº=1.51 V • Fe+3 +3e- Fe(s) Eº=0.036V