AP Chemistry Study Guide, Test 4, Spring 2013 Vocabulary
advertisement

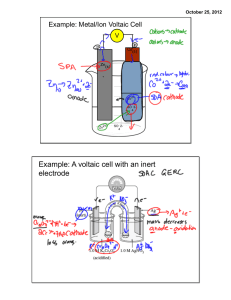
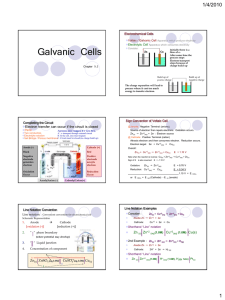
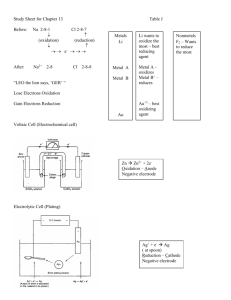
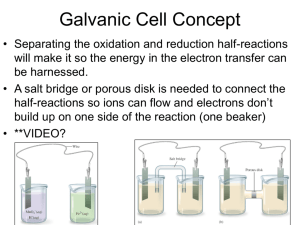
AP Chemistry Study Guide, Test 4, Spring 2013 Vocabulary Oxidation (OILRIG) Reduction Oxidation Number (what is a substances oxidation #) Oxidizing agent Reducing Agent Galvanic/Voltaic Cell Electrolytic Cell Anode (AN OX) Cathode (RED CAT) (Ca+hode) Inert Electrode Salt Bridge Direction of Electron Flow (FATCAT) Porous Disk or Cup Cell Potential Volt (the unit) Standard Conditions (naught) Anion/Cation Standard Cell/Line Notation How can you tell an electrochemical reaction is spontaneous? How can you determine which substance is being reduced/oxidized? Use the chart of standard reduction potentials, or look at the oxidation numbers when given the overall reaction. http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/electroChem/volticCell.ht ml Voltaic cell simulator. 1. Consider the voltaic cell: Cd(s) / Cd2+(aq) // Ni2+(aq) / Ni(s) Write the half-cell reactions and the overall cell reaction. Make a sketch of this cell and label it. Include labels showing the anode, cathode, and direction of electron flow. Calculate E°cell for this galvanic cell and label it as a voltmeter in your sketch. 2. Consider the voltaic cell: Zn(s) / Zn2+(aq) // Cr3+(aq) / Cr(s) Write the half-cell reactions and the overall cell reaction. Make a sketch of this cell and label it. Include labels showing the anode, cathode, and direction of electron flow. Calculate E°cell for this galvanic cell and label it as a voltmeter in your sketch. See text for additional problems: Page 183, #57, 59 Page 868, #14-22, 26, 28, 34, 36