Electrochemistry
advertisement

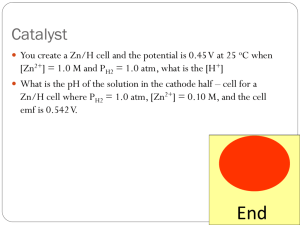
Electrochemistry Chapter 20 The next several topics describe battery cells or voltaic cells (galvanic cells). An electrochemical cell is a system consisting of electrodes that dip into an electrolyte and in which a chemical reaction either uses or generates an electric current. A voltaic or galvanic cell is an electrochemical cell in which a spontaneous reaction generates an electric current. An electrolytic cell is an electrochemical cell in which an electric current drives an otherwise nonspontaneous reaction. Physically, a voltaic cell consists of two half-cells that are electrically connected. Each half-cell is the portion of the electrochemical cell in which a half-reaction takes place. The electrical connections allow the flow of electrons from one electrode to the other. The cells must also have an internal cell connection, such as a salt bridge, to allow the flow of ions. The Cu2+ ions gain two electrons, forming solid copper. The zinc metal atom loses two electrons, forming Zn2+ ions. The electrons flow through the external circuit from the zinc electrode to the copper electrode. Ions flow through the salt bridge to maintain charge balance. A salt bridge is a tube of electrolyte in a gel that is connected to the two half-cells of the voltaic cell. It allows the flow of ions but prevents the mixing of the different solutions that would allow direct reactions of the cell reactants. In the salt bridge, cations move toward the cathode and anions move toward the anode. The electrode at which oxidation takes place is called the anode. The electrode at which reduction takes place is called the cathode. Electrons flow through the external circuit from the anode to the cathode. This figure below illustrates the same reaction, replacing the light bulb with a voltmeter. The solution on the right is now blue from the Cu2+ ion formed. You construct one half-cell of a voltaic cell by inserting a copper metal strip into a solution of copper(II) sulfate.You construct another halfcell by inserting an aluminum metal strip in a solution of aluminum nitrate.You now connect the half-cells by a salt bridge. When connected to an external circuit, the aluminum is oxidized. Sketch the resulting voltaic cell. Label the anode and the cathode, showing the corresponding half-reactions. Indicate the direction of electron flow in the external circuit. e- cathode Cu e- CA2+ NO3- anode Al Cu2+ Al3+ SO42- NO3- Cu2+ + 2e- Cu Al Al3+ + 3e- Voltaic cell notation is a shorthand method of describing a voltaic cell. The oxidation half-cell, the anode, is written on the left. The reduction half-cell, the cathode, is written on the right. The cell terminal is written on the outside: left for the anode and right for the cathode. Phase boundaries are shown with a single vertical bar, |. For example, at the anode, the oxidation of Cu(s) to Cu2+(aq) is shown as Cu(s) | Cu2+(aq). At the cathode, the reduction of Zn2+(aq) to Zn(s) is shown as Zn2+(aq) | Zn(s). Between the anode and cathode the salt bridge is represented by two vertical bars, ||. The complete notation for the reaction is Cu(s) | Cu2+(aq) || Zn2+(aq) | Zn(s) When the half-reaction involves a gas, the electrode is an inert material such as platinum, Pt. It is included as a third substance in the half-cell. For example, the half-reaction of Cl2 being reduced to Cl- is written as follows: Cl2(g) | Cl-(aq) | Pt Because this is a reduction, the electrode appears on the far right. For the oxidation of H2(g) to H+(aq), the notation is Pt | H2(g) | H+(aq) In this case, the electrode appears on the far left. Aqueous Electrolysis For a molten salt, the possible reactions are limited to those involving the ions from the salt. In aqueous situations, however, the possible reactions of water must also be included. 2H2O(l) + 2e- H2(g) + 2OH-(aq) 2H2O(l) O2(g) + 4H+(aq) + 4e- The electrolysis of sodium chloride is the basis of the chlor-alkali industry, which produces chlorine and sodium hydroxide. The chlor-alkali mercury cell uses mercury metal as the cathode. Sodium is reduced, rather than water, forming a sodium mercury amalgam. Metals can also be purified by electrolysis. Here we see copper being purified.