Maxwell-Boltzmann ppt
advertisement

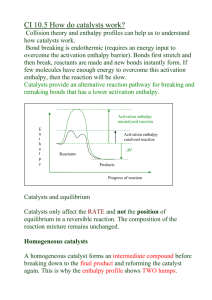
Maxwell-Boltzmann; Temperature and Catalysts Activation Energy • Collisions only result in a reaction if the particles collide with enough energy to get the reaction started. This minimum energy required is called the activation energy for the reaction. • Only a fraction of the total population of a chemical species will be able to collide with enough energy to react (E ≥ Ea) Maxwell-Boltzmann Distribution • In any system, the particles present will have a very wide range of energies. For gases, this can be shown on a graph called the Maxwell-Boltzmann Distribution which is a plot of the number of particles having each particular energy. The Maxwell-Boltzmann Distribution and Activation Energy • Notice that the large majority of the particles don't have enough energy to react when they collide. • To enable them to react we either have to change the shape of the curve, or move the activation energy further to the left. M-B Distribution and Temperature • Increasing the temperature: – increases the average kinetic energy of the gas (slight shift to the right) – Increases the number of particles whose energy exceeds the activation energy Catalysts and Activation Energy • To increase the rate of a reaction you need to increase the proportion of successful collisions. • Catalysts increase the rate of reaction by providing an alternative way for the reaction to happen which has a lower activation energy. M-B Distribution and Catalysts Summary • Maxwell-Boltzmann Energy Distribution Curve – distribution of a population of a chemical by energy – Used to show effect of temperature and catalysts on the rate of a chemical reaction • Temperature – changes average kinetic energy of population – shifts and distorts the M-B Distribution curve – changes the proportion of particles with Ea. • Catalysts – provide alternative pathway with a lower the activation energy – Ea on M-B curve is shifted left – no effect on distribution curve itself