Document
advertisement

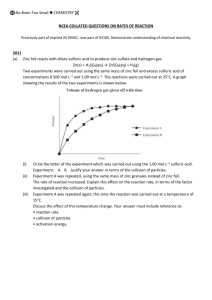
Lesson 6.05 Rate and Collision Theory Introduction • Have you ever driven a bumper car? • The point of driving a bumper car, at least for most people, is to collide with as many other cars as possible. • Imagine that you and your friends are driving the bumper cars at night, and there are no lights on the ride. • This means that all of the drivers will be driving around randomly, and it will be much harder to aim your car at specific victims. • Do you think that the number of bumper cars being driven will affect the number of collisions that you and the other drivers experience? • What about speed? How do you think the number of collisions you and the other drivers experience would change if you increased the speed of all the cars? Keep the relationship between amount, speed, and collisions in mind as you continue into the lesson to discuss the rates of chemical reactions. Objectives After completing this lesson, you will be able to: • Explain how various factors affect the rate of a chemical reaction. • Describe the collision theory as a model of reaction rate. Is it worth it? When chemists plan a reaction, they ask questions like: • Will it produce useful amounts of the desired product? • Will the reaction proceed rapidly enough to be useful? Kinetics • The area of chemistry that studies reaction rates is called kinetics. • The measure of the rate of a reaction involves measuring the change in something over a given amount of time. How is it measured? The rate of a reaction is usually measured in one of two ways: • The decrease in concentration or amount of reactant over time • The increase in concentration or amount of product over time Collision Theory • The idea that in order for compounds, atoms, or ions to react successfully, the particles must collide together. Successful Collisions A successful collision is one that results in making the product(s). Two factors must be true for a reaction to be “successful”: • Sufficient energy • Correct orientation Sufficient Energy • The collision between reactants must have enough energy for the reactants’ valence energy levels to penetrate each other. • This interaction between valence energy levels allows the electrons to rearrange to form new bonds. • If a collision between two reactants does not have enough energy, the collision will not be able to produce the products. Energy Distribution • We can use a graph to represent the distribution of energy within a sample of particles at a given temperature. • This general curve, called a Maxwell-Boltzmann distribution, shows us that the majority of the particles have moderate amounts of energy, near the average kinetic energy of the sample, while some particles have energy higher or lower than that average value. Energy Distribution • Remember that for a reaction to happen particles must collide with energy equal to or greater than the activation energy for the reaction. • That activation energy requirement can be marked on the diagram to show us the amount of a given sample that has the possibility of meeting this energy requirement in a collision. A Maxwell-Boltzmann distribution graph • The y-axis of the graph is labeled “number of particles,” increasing as you go up the axis, and the x-axis is labeled (energy), increasing left to right. • The shape of the graph is like that of a bell or hill, showing that the majority of the particles have a kinetic energy near the average kinetic energy of the sample, while some particles have more energy (far right of the graph) and some particles have less energy (far left of the graph). A Maxwell-Boltzmann distribution graph A Maxwell-Boltzmann distribution graph Correct Orientation • When reactants come in contact with each other during their random movement, the orientation of their collision will determine if the collision is successful The hydrogen chloride molecule is correctly oriented to the double bond in the ethene molecule. Incorrect Orientation These are all incorrect orientations These will not be successful in producing the new product; the two reactants will just “bounce” off of each other. Factors Affecting Reaction Rate- Concentration Increasing Concentration For many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction Be careful: doubling the concentration of one of the reactants doesn’t mean you will double the rate of the reaction. The amount by which an increase in concentration increases the rate of a reaction depends on many different properties of the reaction. Factors Affecting Reaction Rate~ Temperature • temperature is a measure of average kinetic energy • we know that the temperature of a system will affect the number of collisions that have sufficient energy to react Factors Affecting Reaction Rate~ Temperature This direct relationship between temperature and rate is due to two reasons: 1. Increasing the temperature of a system increases the average kinetic energy of the particles. This means more collisions with enough energy to react successfully. 2. Increasing the temperature of a system increases the kinetic energy ,meaning that the particles are moving faster. There are more collisions per minute at a higher temperature then at a lower temperature. More collisions in a given amount of time means there are more opportunities for collisions to meet both requirements for success. Factors Affecting Reaction Rate ~Catalysts • Sometimes it is not possible to raise the temperature to help the reaction move faster • When a catalyst is used, it provides an alternate pathway for the reaction that requires less activation energy. EXAMPLE: Enzymes. In your body, living cells can only survive within a narrow temperature range. Raising the temperature within the cells to speed up the necessary chemical reactions would result in damage to the very cells that the reactions support. Catalysts Maxwell-Boltzmann distribution curve Did You Know? • Some substances, called inhibitors, actually decrease the rate of catalyzed chemical reactions. • They do this by interfering with the catalysts’ ability to participate in the reaction. • Many biological poisons are inhibitors that interfere with the enzymes in your body. Increasing the Rate of a Reaction • Increasing the temperature of a system allows more particle collisions to meet the energy requirement for a successful collision. • Lowering the activation energy requirement of a reaction allows more collisions to be successful without changing any properties of the collisions themselves. Adding a catalyst! Increasing the Rate Some other ways to increase the rate of a reaction that you have already learned about include: • Agitation (stirring or mixing) • Increasing the surface area (making the particles smaller) Congratulations! You should now be able to: • Explain how various factors affect the rate of a chemical reaction. • Describe the collision theory as a model of reaction rate. Please review this lesson again if you need to