The Gas Laws
advertisement

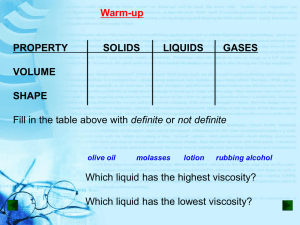
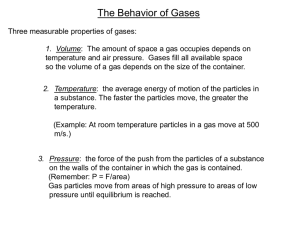
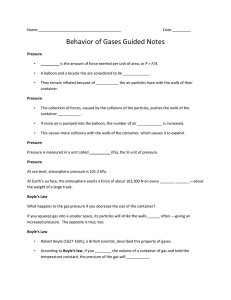
THE GAS LAWS •Define pressure and gas pressure. •Identify factors that affect gas pressure. •Predict changes in gas pressure due to changes in temperature, volume, and number of particles. •Explain Charles’s law, Boyle’s law, Gay- Lussac’s Law and the combined gas law. •Apply gas laws to solve problems involving gases. PRESSURE = IS THE RESULT OF A FORCE DISTRIBUTED OVER AN AREA. The SI unit of pressure is derived from the units of force and area. The SI unit for pressure is Pascal (Pa). Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas. FACTORS THAT AFFECT GAS PRESSURE 1. Temperature = Raising the temperature of a gas will increase its pressure if the volume of the gas and the number of particles are constant FACTORS THAT AFFECT GAS PRESSURE 2. Volume= Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are constant. FACTORS AFFECT GAS PRESSURE 3. Number of Particles = increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant. GAS LAWS Charles’s Law = Boyle’s Law = Gay-Lussac’s Law = (pressure is constant) P1V1 = P2V2 (temperature is constant) P 1 = P2 T1 (volume is constant) T2 Combined Gas Law = P 1V1 = P2 V2 T1 T2 Gas Laws variables and units Factors affecting the behavior of a gas pressure volume temperature Variable Unit Gas Laws variables and units Factors affecting the behavior of a gas Variable pressure Unit Pa atm P psi volume V L cm3 temperature T K 0C