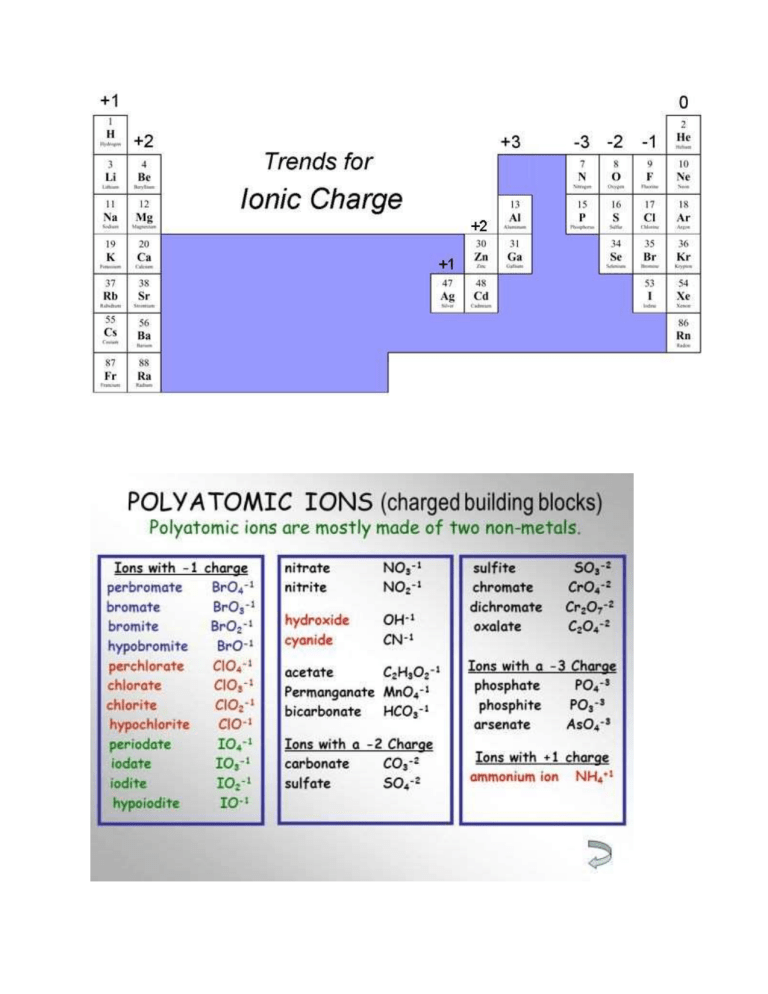
Write the equilibrium equation for the following ionic compounds (don’t forget charges) 1) MgCl2 2) Mg3N2 3) KN3 4) CaSO3 5) Ba3(PO4)2 Finding molecular solubilities from Ksp with the balanced ionic equilibrium reaction Ksp = [product1]coefficient of balanced P1 in balanced eqn [product2] coefficient Ex. AgCl(s) -- Ag+(aq) + Cl-(aq) Ksp = [Ag+] [Cl-] Ex. Mg(OH)2 - Mg2+(aq) + 2OH-(aq) Ksp = [Mg2+] [OH-]2 *solids and liquids are not included in Ksp. Only (aq) – aqueous* Ex. Mg(OH)2 - Mg2+(aq) + OH-(aq) The Ksp of Mg(OH)2 1.6 x 10-12 find the concentration of Mg ions in the solution 1) Balance the ionic equation Ex. Mg(OH)2 - Mg2+(aq) + 2OH-(aq) Mg -1 Mg -1 OH – 2 OH - 2 2) Create an ICE (Initial, Change, Equilibrium) table (I’m not sure if this is taught, this is just how I do it and I think that it will help with understanding) - We do not do this for the reactants. Only for the products - The initial (I) values for the products (right side) is normally 0 for these types of problems - The concentration (C) values depends on the coefficients of the balanced equilibrium equation. Since the Mg was not changed, we use +x. Since the coefficient OH was changed we write +2x (or whatever you changed the coefficient to) - For the equilibrium (E) values, we simply add up the I and the C values of the products Mg(OH)2 - Mg2+(aq) + 2OH-(aq) I 0 0 C +x +2x E x 2x 3) Write out the Ksp equation Ksp = [Mg2+] [OH-]2 4) Solve the equation mathematically in terms of x with the revised ICE table 1.6 x 10-12 =[x] [2x]2 a. Remember when we multiply, we add exponents. Don’t forget to distribute the two over 1.6 x 10-12 = 4x3 b. divide the Ksp value by 4 and take the cubed root 4 x 10-13 =x3 On a calculator, ^ then, 1 (Ab/c), 3 and then = 7.4 x 10-5 M Mg If we were solving for OH- ions, we would multiply this value by 2 Note: this is just how I learned how to solve these types of problems, if you do not feel comfortable making the ICE table, that is completely fine 😊 I used this youtube video to write this. He explains it very well. https://www.youtube.com/watch?v=cABKIN5eiSQ This is also a good video https://www.youtube.com/watch?v=tUnbm379Wfk Finding Ksp when given a concentration Ex. The solubility of PbBr2 is 0.012M at 25*C. find the Ksp 1) Find the charges of the ions and balance appropriately Note: Pb is a transition element which does not have a predictable charge like the other groups of elements, but we can figure out its charge by knowing the charge of Br. Since Br is in the halogen group it would have a -1 charge and there is two of them so this gives us a -2. In order for the compound to have a neutral charge, Pb would therefore have to be a +2 - PbBr2 (s) - Pb2+ + 2BrPb+2 – 1 Pb – 1 Br-1 – 2 Br - 2 2) Create an ICE table like in the previous example based on the balanced equation PbBr2 (s) - Pb2+ + 2BrI 0 0 C +x +2x E x 2x 3) Write the Ksp equation Ksp = [Pb2+] [Br-]2 4) Put the x values from the ICE table into the Ksp equation Ksp = [x] [2x]2 5) Substitute the value for the concentration (0.012) in for x and solve using order of operations a. Note: cube the 0.0012 then multiply by 4 Ksp = 4(0.012)3 Ksp = 6.9 x 10-6






