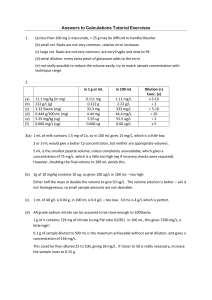
Demonstration: Ksp, Solubility, and Precipitate Formation _______________________________ formation.
advertisement

Demonstration: Ksp, Solubility, and Precipitate Formation Purpose: To observe the relationship between the _________ value of an ionic compound and _______________________________ formation. Procedure: Various concentrations of Silver Nitrate and Sodium Chloride Solutions will be mixed together to observe whether or not a ppt forms. Comparison of the calculated _______ value to the accepted ________ value for ______________________________________ will allow us to predict whether or not a ppt should form. Write the Equilibrium Equation for the Dissolution of solid _____________________. ________________________ (s) ___________ (aq) + ___________ (aq) Ksp=___________________ Write the expression for Ksp 10.0 mL of _____________________________ will be added to 10.0 mL of ______________________________. Will a ppt form? To answer this question, we must calculate __________. So we must calculate the _________________________ of _________ and _________ after __________________. A) Original Solutions are Mixed B) Dilution #1 (10x) C) Dilution #2 (100x) D) Dilution #3 (1000x) E) Dilution #4 (10000x)