powerpoint
advertisement

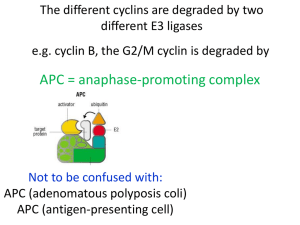
Cell-Cycle Regulation and the Genetics of Cancer Outline The control of cell division Normal cell cycle Yeast as a model organism Cell cycle control: Molecular Mechanisms Regulation of cyclin-CDK activity Checkpoints that regulate passage through cell cycle The normal cell division Cyclin-dependent kinases (CDKs) collaborate with cyclins to ensure the proper timing and sequence of cellcycle events Experiments with yeast helped identify genes that control cell division Properties of yeast Grow as haploid or diploid organisms Can identify recessive mutations in haploids Complementation analysis in diploids Budding – daughter cell arises on surface of mother cell and grows in size during cell cycle. Helps determine stage of cell cycle. Isolation of temperaturesensitive mutants in yeast Mutants grow normally at permissive temperature Mutants loses gene function at restrictive temperature Thousands of cell cycle mutants have been identified A cell-cycle mutant in yeast (a) growth at permissive temperature displays buds of all sizes (b) growth at restrictive temperature shows cells have finished first cell cycle and arrested in the second A double mutant reveals which mutation is needed Human CDKs and cyclins can function in yeast in place of native proteins 70 cell-cycle genes identified through temperature-sensitive mutation screens Cell Cycle Control: Cyclin-dependent kinases (CDK) and their regulatory subunits, the cyclins Cyclins •50-90kDa proteins with conserved ‘cyclin box’ region •Forms 5 alpha helices. •Conservation between Rb and TFIIB suggests that cyclin box domain regulates protein interactions related to cdk regulation and transcription Cyclin – CDK interactions Cdks ( blue) by themselves are inactive. Activation occurs through phosphorylation of the T loop (green) and the binding of cyclin (purple) at the PSTAIRE helix (red). These events lead to a conformational change that produces a functional active site (yellow) How enzymes select their substrate a, b, In general, enzymes recognize their targets through structural complementarity between the substrate and the enzyme's active site (indicated here by the shape of the 'pocket'). Small substrates (a) and relatively small modification sites on proteins (b) can be recognized by this mechanism. c, Some enzymes make additional, specific contacts with the substrate that enable them to distinguish between proteins that have identical or related sites of modification. d, cyclin-dependent protein kinases (CDKs) have relegated that function to the exchangeable cyclin subunit, enabling a single CDK catalytic subunit to exist in numerous forms with different specificities. Cell cycle: cyclin guides the way by C Wittenberg Nature 434, 34-35 (3 March 2005) Higher eukaryotes have more forms of both cyclins and CDKs compared to lower eukaryotes. CDK1 Cyclin-dependent kinases (CDKs) control the cell cycle by phosphorylating specific serine and threonine residues of select proteins during different phases of the cell cycle other proteins E.g. Nuclear lamins CDK substrates Underlie inner surface of the nuclear membrane Probably provide structural support for nucleus May also be site for assembly of DNA replication, transcription, RNA transport, and chromosome structure proteins Dissolution of nuclear membrane during mitosis is triggered by CDK phosphorylation of nuclear lamins REGULATON OF CYCLIN–CDK ACTIVITY 1. Cyclin availability Association with a cyclin is absolutely required for Cdk activity. Cyclin levels can be changed by transcriptional regulation and/or by ubiquitin-dependent proteolysis. E.g. cyclins D and E contain a PEST sequence [segment rich in proline(P), glutamic acid (E), serine (S) and threonine (T) residues]: which are required for efficient ubiquitin-mediated cyclin proteolysis at the end of a cell cycle REGULATON OF CYCLIN–CDK ACTIVITY 2. Inhibitory phosphorylation Cyclin–Cdk complexes can also be inactivated by phosphorylation of tyrosine and threonine residues close to the active site of the Cdk subunit. This phosphorylation is mediated by Wee1-type protein kinases, and the inhibitory phosphate groups are removed by Cdc25-type phosphatases REGULATON OF CYCLIN–CDK ACTIVITY 3. Stoichiometric inhibition CDK activity can be regulated by stoichiometric inhibitors (cyclin kinase inhibitors-CKIs), which bind to CDK alone or to the CDK-cyclin complex and regulate CDK activity. Lower eukaryotes possess a single CycB–Cdk1 specific inhibitor, whereas in higher eukaryotes 2 distinct families exist; the INK4 family and Cip/Kip family REGULATON OF CYCLIN–CDK ACTIVITY 3. Stoichiometric inhibition… CKIs are regulated both by internal and external signals: E.g. expression of p21 is under transcriptional control of the p53 tumour suppressor gene (internal), whereas the expression and activation of p15 and p27 increases in response to transforming growth factor b (TGF-b), contributing to growth arrest (external) REGULATON OF CYCLIN–CDK ACTIVITY 4. Intracellular localisation Intracellular localization of different cell cycleregulating proteins also contributes to CDK regulation Checkpoints integrate repair of chromosome damage with events of cell cycle G1-S checkpoint p53 – transcription factor that induces expression of DNA repair genes and CDK inhibitor p21 p53 pathway activated by ionizing radiation or UV light (causing DNA damage) during G1 phase delays entry into S phase DNA is repaired before cell cycle continues If DNA is badly damaged cells commit suicide (programmed cell death or apoptosis) G1-S phase transition Mutations in p53 disrupt G1-S transition Gene amplification in tumour cells that appear as homogenously staining regions (HSR) Small chromosome-like bodies (called minutes) in tumour cells that lack centromeres and telomeres p53 mutants do not induce p21 and cell cycle is not arrested Cells replicate damaged DNA Cells die or DNA is degraded and cell is engulfed and digested by neighboring cells (apoptosis, or programmed cell death) S phase checkpoint Individuals affected by ataxia telangiectasia (AT), an autosomal recessive disorder, are unable to slow down DNA replication after exposure to radiation. The AT gene (ATM) codes for a protein kinase with homology to the catalytic domain of phosphatidylinositol 3kinase (PI-3 kinase) and serves as a checkpoint gene in response to DNA damage. ATM is central to dsb responses. G2-M transition is controlled by phosphorylation and dephosphorylation Two checkpoints act at the G2-M transition double strand breaks Checkpoint in M spindle damage Checkpoints ensure genomic stability Defective checkpoints Chromosome aberrations Aneuploidy Changes in ploidy Single-stranded nicks – normally repaired in G1 phase Chromosome loss or gain – normally corrected in G2-M checkpoint Three classes of error lead to aneuploidy in tumor cells Normal cells Cancerous cells General reading: MBoC by Alberts et al (4th ed): pgs 863-906 OR Cancer Biology by RJB King : pgs 148 - 158……..OR Chapter 9 Mol & Cell Biol of Cancer by Knowles and Selby Optional reading: The cell cycle: a review…. targets in cancer by K Vermeulen, DR. Van Bockstaele and ZN. Berneman Cell Proliferation (June 2003) 36(3) pp131-149. Cell cycle by Gary S Stein et al www.els.net