Presentation
advertisement

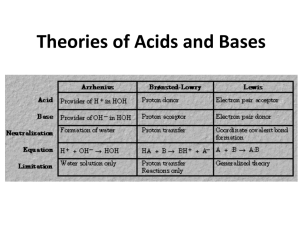
Unit 12 Weak Acids and Weak Bases What makes a Weak Acid? • Weak acids (covalently bonded H compounds) are ionized by water less than 100% • HBrO + H2O H3O+ + BrO• According to Bronsted-Lowry, hypobromous acid is the acid and the hypobromite ion is the conjugate base. What makes a Weak Base? • Weak bases are the conjugate bases of the weak acids (the anions) or they are the organic bases—the amines • The amines are derivatives of ammonia • NH3 + H2O NH4+ + OH• For Bronsted-Lowry, ammonia is the base (proton acceptor) and the ammonium ion is the conjugate acid (proton donor) Weak: Don’t ionize 100% • Bronsted-Lowry model for Acid-Base Reactions • HA + H2O D H3O+ + A• HA acid and A- conjugate base • B + H2O D BH+ + OH• B base and BH+ conjugate acid Conjugate Acid/Base Conjugate Acid Conjugate Base HF F- HSO4- SO42- NH4+ NH3 Examples • Label the acid, base, conjugate acid, and conjugate base for #2 on page 374 • Determine it if substance would act as an acid or base for #4 on page 374 • **remember acids lose an H+ and bases gain an H+ Examples • On page 375 let’s look at number 26 Homework 1 • Page 374 (1,3,5, 25) Equilibrium Expression • For HA + H2O H3O+ + A• Ka = [H+] [A-] / [HA] • For Base + H2O BH+ + OH- • Kb = [BH+ ] [OH-] / [Base] EQUILIBRIUM • Weak acids and bases set up an equilibrium situation. • EQUILIBRIUM: the rate of the forward rxn = rate of the reverse rxn • K is the equilibrium constant • Ka = [H+]*[A-] / [HA] • Kb = [BH+] * [OH-] / [B] Weak Solutions • If you put a weak acid in water, the water pulls H+ from the acid because the covalent bond is weaker in the acid. • [H+] = [A-] • If you put a weak base in water, the weak base pulls an H+ from the water. • [OH-] = [BH+] Example: Weak Acid • Calculate the pH of a 2M HBrO solution. What is the % ionization? Example: Weak Base • What is the pH of a 3M solution of ammonia, a weak base? Example: Finding Ka • Aspirin is a weak acid. A 0.1 M solution has a pH = 2.24. What is the Ka? Example: Finding Ka • Saccharin is a weak acid. A 0.1 M solution ionizes 22%. Calculate the concentration of all species, the pH, and Ka. Example: Finding Kb • Cocaine is a weak base. A 0.0010 M solution has a pH of 9.7, what is the Kb? Homework 2 & 3 • Page 375 33-41 odd • Page 376 49, 53-58 Polyprotic Acids • Water pulls off 1 H+ at a time. • Ionization occurs in stages • Water will be less effective at pulling off an H+ from the negative anion at each step. • The #H+ = # ionizations Multiprotic Acids • H3A H+ + H2A• H2A- H+ + HA2• HA2- H+ + A3- Ka1 Ka2 Ka3 Multiprotic Acid Example • What is the pH and concentration of all species present in a 1.5M solution of phosphoric acid (in soft drinks)? Homework 4 • Page 375 (45-48) How to determine if the salts are acidic, basic or neutral in water solutions? • • • • • NaNO2 NaF HCl NaCN HCO3- Homework 5 • Page 376 (59, 60)