Worksheet 1 9/5/13
advertisement

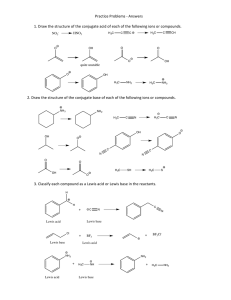
SI Chem 231 Leader: Kelsey Worksheet 1 Chapter 1 Review: Fill in any unshared electrons that are missing from these structures: Is this formula correct? Why/why not? C2H7 Identify the most electronegative element in each of the following molecules: CH2FCl HOCH2CH2NH2 FCH2CH2CH2Br CH3OCH2Li Propose a structure that: a) Contains 2 sp2 carbons and 2 sp3 carbons b) Contains 4 carbons, all are sp2 c) Contains 2 sp carbons and 2 sp2 carbons Define: Bronsted-Lowry acid: Bronsted-Lowry base: Strong acid: Lewis acid: Lewis base: The acidity constant, Ka, gives the exact strength of a given acid HA in a water solution. HA + H20 A- + H30+ Ka= [H30+] [A-] [HA] How would you write the Ka for: HCl + H20 H3O+ + ClKa= Acid strengths are normally given using pKa values, rather than Ka values: pKa= Circle one: A stronger acid has a (smaller/larger) pKa and a (smaller/larger) Ka. A weaker acid has a (smaller/larger) pKa and a (smaller/larger) Ka. Fill in the blank: A strong acid yields a A weak acid yields a Which acid is stronger in each pair? H3PO4 Phosphoric Acid pKa= 2.16 HCl Hydrochloric Acid pKa=-7.0 H2O HCCH Water pKa=15.74 HNO3 Nitric Acid pKa=-1.3 Acetylene pKa=25 CH3CO2H Acetic Acid pKa=4.76 Using curved arrows, show how acetaldehyde, CH3CHO, can act as a Lewis base in a reaction with a strong acid, H+.