electrons - Seattle Central College
advertisement

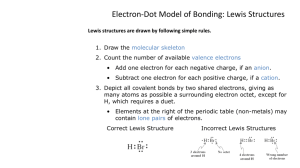
Chapter #10 Chemical Bonding CHAPTER 12 Forces Between Particles Noble Gas Configurations Ionic Bonding Covalent Bonding VSEPR Theory and Molecular Geometry Electronegativity Polar Bonds and Molecules Atomic Stability It has been recognized for a long time that the noble gases have great chemical stability. With few exceptions they are unreactive or inert. The noble gases have 8 valence electrons with the exception of He which has 2. He Ne Ar Kr Xe 1s2 1s22s22p6 1s22s22p63s23p6 1s22s22p63s23p64s23d104p6 1s22s22p63s23p64s23d104p65s24d105p6 Lewis Diagrams The electronic configuration of the noble gases is described as being energetically stable. We can draw a Lewis diagram to illustrate the number of valence electrons an atom has. In a Lewis diagram valence electrons are represented by dots placed above, below and to the left and right of the atoms symbol. e.g. element with 4 valence electrons E Lewis Diagrams There are two simple rules to keep in mind when drawing Lewis diagrams: • Place one dot in each of the four locations before doubling up. • There can be only a maximum of 2 dots in any one location. E E E E Lewis Diagrams What is the Lewis diagram for H? 1. First write the electron configuration: 1s1 2. Identify the number of valence electrons. 1 valence electron. H For a representative element it is easy to identify the number of valence electrons as this is equal to the group number. Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons Lewis Diagrams What is the Lewis diagram for S? 1. First write the electron configuration: [Ne]3s23p4 2. Identify the number of valence electrons. 6 valence electrons S Alternatively you can recognize that S is in group VIA so has six valence electrons LEWIS STRUCTURES OF THE ELEMENTS 1 2 13 14 15 16 17 18 He H Be B C N O F Ne Na Mg Al Si P S Cl Ar Li LEWIS STRUCTURES OF IONS (AFTER REMOVAL OR ADDITION OF ELECTRONS) 1 1+ 2 H 2+ Li Be Na Mg 13 B 3+ Al 14 15 16 17 18 4- 3- 2- 1- He C N O F Ne Si P S Cl Ar Lewis Diagrams The octet rule states that: “Atoms interact in order to obtain a stable octet of eight valence electrons” The octet rule works extremely well at describing the interactions of the representative elements. Lewis Diagrams One way in which atoms can interact to satisfy the octet rule is by transferring electrons between each other. Transferring of electrons results in the atoms acquiring net positive and negative charges. When an atom loses or gains electrons a simple ion is formed. Cations have more protons than electrons and are positive. Anions have more electrons than protons and are negative. Ion Formation Consider a Na atom what happens if it loses one electron? Na I.E. Na+ 1e- + [Ne] [Ne]3s1 11 P and 10 e- 11 P and 11 e- Consider a Cl atom would you expect it to lose or gain electrons? Cl [Ne]3s23p5 17 P and 17 e- + 1e- E.A. Cl[Ne]3s23p6 17 P and 18 e- Ion Formation Metals tend to lose electrons forming positively charged ions called cations. • A representative metal will lose its group number of electrons to obtain a stable octet. Na → Na+ Mg → Mg2+ + + 1e- ( Isoelectronic with Ne) 2e- (isoelectronic with Ne) What would the charge be of the ion formed by a Li atom? And which Noble gas is it isoelectronic with? +1 The ion formed would be Li+ Isoelectronic with He Ion Formation Non-metals tend to gain electrons forming negatively charged ions called anions. • A representative non-metal will gain (8 - group number) electrons to obtain a stable octet. O + 2e→ O2- (isoelectronic with Ne) S + 2e→ S2- (isoelectronic with Ar) What would the charge be of the ion formed by a I atom? Which Noble gas is it isoelectronic with? -1 The ion formed would be IIsoelectronic with Xe Lewis Structure of NaCl + + + Na Cl Na Cl Na Cl Cl- Na+Cl-Na+Cl-Na+ Forces between oppositely charged ions are called Ionic bonds. Each ion is surrounded by an octet of Electrons, thus making the ions stable. Crystal Lattice of NaCl Ionic compounds do not exist as discrete molecules. Instead they exist as crystals where ions of opposite charges occupy positions known as lattice sites. Ions combine in the ratio that results in zero charge to form ionic compounds. Which ions are the smaller ones? Crystal Lattice of NaCl Crystal Lattice of NaCl Ionic compounds do not exist as discrete molecules. Instead they exist as crystals where ions of opposite charges occupy positions known as lattice sites. Ions combine in the ratio that results in zero charge to form ionic compounds. Which ions are the smaller ones? Sodium Crystal Lattice of NaCl Molecular Compounds In our early lectures we defined a molecule as “as a compound made of nonmetals.” Molecules exist as particles containing the number of atoms specified by their formula. e.g. a water molecule is a particle containing 2 hydrogen atoms and one oxygen atom and has the formula H2O. Molecular Compounds Non-metals may also complete their octets by sharing electrons. This may occur between non-metal atoms of the same type: e.g. H2, O2, N2, Cl2, F2, I2, etc Or between different types of non-metal atoms: e.g. CO2, H2O, CH4, etc Molecular Compounds Consider two hydrogen atoms separated by a large distance. Each has 1 electron in a 1s atomic orbital. - - + + Why does the electron stay around the nucleus? Now lets bring the two atoms together so there orbitals overlap. Molecular Compounds - + + - The atomic orbitals overlap to form a new molecular orbital. This is a stable configuration as each H atom can have a full 1s subshell (like He) where the electrons spend most of their time shared between the atoms. In this arrangement each nucleus feels an inwards attraction to the two electrons. This is called covalent bonding. Molecular Compounds - + + - This new arrangement of protons and electrons is more stable than separate hydrogen atoms since the attraction of a proton to two electrons is a stronger attraction compared to one proton to one electron of a hydrogen atom. Molecular Compounds We can draw Lewis diagrams showing the arrangement of valence electrons in covalent compounds. In these diagrams we represent each pair of electrons between atoms as a line. So for the H2 molecule discussed previously the Lewis diagram would be: H–H All other electrons are represented by dots as described previously. Lewis Structures Draw Lewis Structures of the following molecular compounds H H Nonbonding electons a. H2O O H H O Note each element has a Noble gas structure by electron sharing b. NH3 H N H HN H H H Covalent bonding e’s Simplified Lewis Structures Straight lines are used to indicate a shared pair, or a covalent bond. H O H Nonbonding electrons Lewis Structure Construction Step 1 Step 2 Step 3 Step 4 Step 5 Connect each element with a single line Use the “P” formula to determine extra bonds Insert the extra bonds, to make double or triple bonds. Give each atom an octet of electrons, except hydrogen Determine the formal charge of each element P = 8(n-q) +2q - 2(n-1) - v N = number of atoms in molecule Q = number of hydrogen atoms V = total number of valence electrons Examples: Give Lewis Structures for the following CO2 H2CO3 SO3 NO2+ Lewis Structure of Carbon Dioxide First, connect atoms with lines O C O Lewis Structure of Carbon Dioxide First, connect atoms with lines O C O Second, use “p” formula to determine the number of extra bonds. P = 8(n-q) + 2q – 2(n-1) - v P = 8(3-0) + 2(0) – 2(3-1) - 16 P = 24 + 0 – 4 - 16 P=4 4 extra bonding electrons 2 extra bonds 2 extra lines Lewis Structure of Carbon Dioxide Third, add extra lines (Three possible locations) O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines (three possible locations) O C O O C O O C O Fourth, give each atom an octet of electrons Lewis Structure of Carbon Dioxide Third, add extra lines (three possible locations) O C O O C O O C O Fourth, give each atom an octet of electrons O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines (three possible locations) O C O O C O O C O Fourth, give each atom an octet of electrons O C O O C O O C O Fifth, give each an atom a formal charge Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O C O O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O C O O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O C O O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive - O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O O C O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive - + O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O O C O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive - - + O C O O C O O C O Lewis Structure of Carbon Dioxide Third, add extra lines O C O O C O O C O Fourth, give each atom an octet of electrons O O C O C O O C O Fifth, give each an atom a formal charge If the element has more than its valence, then it is negative If the element owns less than its valence, then it is positive - O C O + - + O C O O C O Lewis Structure of SO3 There are actually three possible Lewis structures for SO3. - O S O - O - O O S O - - O S - O O Each of these three structures is equivalent. We say they are in “resonance” or that they are “resonance structures”. Resonance demonstrates how the loosely held pi electrons are free to move about. Or another way to look at it is that the pi electrons are spread over the entire molecule, making it more stable Molecular Geometry So far we have been considering how electrons are distributed between atoms in molecules and polyatomic ions. An important question is: How can we predict the shape of molecules and polyatomic ions? Molecular Shape The simplest polyatomic ion or molecule is made of two atoms: What is the shape of this type of molecule or ion? The only one way to join two atoms is with a line. All diatomic molecules and ions have a linear geometry. Molecular shape is the geometry defined by the atoms making up the molecule. Predicting Molecular Shape Valence shell electron pair repulsion theory (VSEPR theory) allows us to predict the 3 dimensional shape of molecules and polyatomic ions with >2 atoms. VSEPR theory states that electrons in lone pairs and bonds move as far away from one another as possible to minimize repulsive interactions. Repulsion Angles Predicting Molecular Shape For a central atom with three electron regions there are two possibilities. Predicting Molecular Shape For a central atom with four electron regions there are three possibilities. Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O 3 regions H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O 3 regions H O H H N H H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H 3 regions 4 regions H Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H 3 regions 4 regions Predicting Molecular Shape To determine the correct shape of a molecule we must first begin with the correct Lewis structure. We then need to determine how many regions of electron density are around the central atom. This is the number of bonds and electron pairs. O S O H O H H N H H 3 regions 4 regions 4 regions Predicting Molecular Shape The arrangement of outer atoms is determined by how many nonbonding pairs there are around the central atom. If there are 2 or less electron regions then the arrangement will always be linear. The situation becomes more complex if there are more than 2 electron regions around the central atom. Predicting Molecular Shape Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 Predicting Molecular Shape Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. Predicting Molecular Shape Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. N N P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) -10 P = 4 , 2 extra lines, right? Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. N N P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) -10 P = 4 , 2 extra lines, right? Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. N N N N P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) 10 P = 4 , 2 extra lines, right? Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. N N N N N N Adding nonbonding pairs P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) -10 P = 4 , 2 extra lines, right? Adding extra bonds Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) -10 P = 4 , 2 extra lines, right? N N N N Adding extra bonds N N Adding nonbonding pairs Consider either nitrogen to be the central atom. Notice there are two volumes of space for electrons Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 First we will start with the Lewis structure of nitrogen. N N N N N N P = 8(n-q) + 2q -2(n-1) – v P = 8(2-0) + 2(0) -2(3-1) -10 P = 4 , 2 extra lines, right? Adding extra bonds Adding nonbonding pairs Consider either nitrogen to be the central atom. Notice there are two volumes of space for electrons Two volumes of electrons repel to 180°, thus linear shape Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 Now consider the Lewis Structure of water. H O H Molecular Shape of Water Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 Now consider the Lewis Structure of water. H O H In water there are two bonding and two nonbonding electron pairs Molecular Shape of Water Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 H O H Nonbonding electrons H H In water there are two bonding and two nonbonding electron pairs. Molecular Shape of Water Use VSEPR theory to determine as much as possible about the structure of N2, H2O, SO3, CH4, NH4+ and NH3 H O In water there are two bonding H and nonbonding electron pairs Nonbonding electrons Bent or v-shape, bond angles less than H H 109.5° Electronegativity Electronegativity is an atoms affinity for electrons. Affinity is a Greek word for loving. Since electrons are attracted to the nucleus, then it makes sense that small atoms with more protons should attract electrons stronger. Therefore, we should predict electronegativity should increase from left to right and bottom to top on the Periodic Chart. Electronegative Chart Molecule Polarity • A molecule will be polar if – it has polar bonds, and – its centers of partial positive and partial negative charges lie at different places within the molecule • Carbon dioxide, CO2, has two polar bonds but, because of its geometry, is a nonpolar molecule Water • Water, H2O, has two polar bonds and, because of its geometry, is a polar molecule center of partial positive charge is midway between the two hydrogen atoms O H + H Water (a polar molecule) Ammonia • Ammonia, NH3, has three polar bonds and, because of its geometry, is a polar molecule center of partial positive charge is midway between the three hydrogen atoms N H H + H Ammonia (a polar molecule) Dichloromethane • Both dichloromethane, CH2Cl2, and formaldehyde, CH2O, have polar bonds and are polar molecules - - Cl + Cl C HH D i ch lorome th an e O- C+ H H Form ald eh yd e The End CHAPTER #10