chapter 12 notes
advertisement

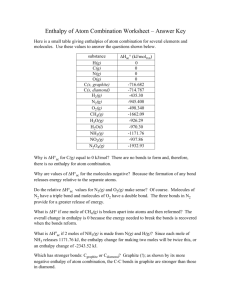
Chapter 12: Heat in Chemical Reactions 12-1 Chemical Reactions that Involve Heat • Chemical reactions involve breaking or making bonds and rearranging atoms. • Breaking bonds requires energy and making bonds releases energy. • Heat- the energy that is transferred from one object to another due to a difference in temperature • Thermochemistry – study of the changes in heat in a chemical reaction. • Exothermic reactions – reactions that RELEASE heat • Endothermic reactions – reactions that ABSORB heat Exothermic reactions: • Burning of a camp stove • C3H8 + 5O2 3CO2 + 4H2O + 2043 kJ • 2043 kJ of heat are released when 1 mole of C3H8 is burned. • The energy released during forming new bonds is greater than the energy required to break the old bonds. Endothermic Reactions: • A process in industry fuel called water gas, passing steam over hot coals • C + H2O + 113KJ CO + H2 • The energy released as new bonds are formed in the products is less than the energy required to break the bonds in the reactants. This energy must be provided for the reaction to take place. The energy provided is stored in the bonds of the products. Calculations • Enthalpy change ∆H • ∆H = Hproducts – Hreactants • Standard Temp and pressure • 1 atmosphere is p 25C is T • Sign ∆H • + • - Process Endo Exo Heat Absorbed Released 12-2 Heat and Enthalpy Changes • Enthalpy – the heat absorbed or gained during a chemical reaction. Accounts for the kinetic energy plus the volume and temperature of the substance. • The difference between energy and enthalpy is very small. When the pressure remains constant, the heat absorbed or released during a chemical reaction is equal to the enthalpy change for the reaction. • How much heat will be released if 1.0g of H2O2 decomposes in a beetle to produce the steam spray • 2H2O2 2H2O + O2 ∆H = -190KJ • 1.0g H2O2 x 1mol H2O2 = 0.029 mol H2O2 • 34 g • 0.029 mol H2O2 x -190kJ = -2.8KJ 2 mol H2O2 12-3 Hess’s Law • Allows you to find enthalpy changes of reactions that cannot be done directly • Hess’s Law- if a series of reactions are added together, the enthalpy change for the net reaction will be the sum of the enthalpy changes for the individual steps. • Consider the haze in a large city. • • • • N2 + 2O2 2NO2 N2 + O2 2NO ∆H = +181KJ 2NO + O2 2NO2 = ∆H2 = -113KJ N2 + 2O2 + 2NO 2NO + 2NO2 • ∆Hnet = ∆H1 + ∆H2 • ∆H = 181 + -113. • ∆H = 68KJ • Rules for applying Hess’s Law • Multiply the enthalpy change by the coefficient of the substance. • If the equation is reversed, so is the sign of ∆H. • Calculate ∆H for the reaction that produces SO2 • S + O2 SO2 • 2SO2 + O2 2SO3 ∆H = -196KJ • 2S + 3O2 2SO3 ∆H = -790 KJ • • • • • • • • 1st switch the 1st reaction. 2SO3 2SO2 + O2 = ∆H = 196KJ 2S + 3O2 2SO3 ∆H = -790KJ You’re left with 2S + 2O2 2SO2 ∆H = (+196KJ) + (-790KJ) ∆H = -594KJ HOWEVER, the equation is only ½ of this so you have to • ∆H = ½ (-594 KJ) = -297 KJ 12-4 Calorimetry • Calorimetry- the study of heat flow and heat measurement • Heat capacity – the amount of heat needed to raise the temperature of the object by 1 Celsius degree. • For example, the heat capacity of a cup of water at 18o C is the number of joules needed to make it 19. • Depends on mass and composition • Specific heat – the heat capacity of 1 gram of a substance • Water = 4.184 J/g C • To raise the temp of 1 gram of water 1 degree C you need 4.184J • Determine ∆H for the rxn. • NaOH Na+ + OH• The calorimeter is filled with 75.0g water. The initial temp is 19.8C. A 0.050-mole sample of solid NaOH is added and the temp increases to 26.7 C. • the temp increased so its exothermic. • The heat lost by the sodium is gained by the water. q is used to show heat. • so we can say qrxn = -qsur • • • • • • • • • • qsur = m x C x (Tf – Ti) m = mass of water C = specific heat temp change = T – T qsur = (75.0g)(4.184J/gC)(26.7 – 19.8) qsur = +2170J (J to KJ) qrxn = -qsur = -2.17kJ ∆H = -2.17kJ 0.050 mole NaOH -43kJ • When a 4.25g sample of solid NH4OH dissolves in 60.0g of water, the temperature drops from 21.0C to 16.9C solve ∆H • NH4NO3 NH4+ + NO3- • • • • • 1st calculate qsur Qsur = m x C x ∆T Qsur = 60.0g x 4.184J/gC x (16.9-21.0) Qsur = -1.0 x 103 J Qrxn = -qsur = + 1.0 x 103 J/1000 = 1 KJ • 4.25g NH4OH x 1 mole NH4OH • • = + 8.5KJ 1.0 KJ 35 g NH4OH 0.121mNH4OH