Chapter 5 - A Level Notes
advertisement

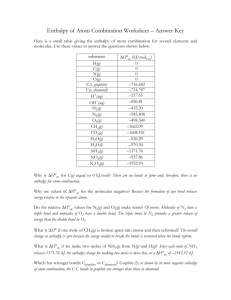
CHEM 1 Enthalpy Changes and Standard Conditions Enthalpy – The term used to describe the heat energy content of a system. It has the symbol H and is measure in kilojoules. Enthalpy changes – ΔH. The heat change measured under conditions of constant pressure, calculated by Enthalpy of reactants – enthalpy of products. Exothermic reaction – release heat energy into the environment. ΔH is always negative. Endothermic reaction – absorb heat energy. ΔH is always positive. Standard conditions - ΔHθ. 100kPa, substances are in their standard states; 298 K. The value (in kJ) of ΔH is given per mole of substance. Standard enthalpy change of formation – ΔHfθ. Enthalpy change when one mole of a compound is formed from its elements in their standard states. Enthalpy of formation of elements – by definition the standard enthalpy f formation of an element in its standard state is zero. Standard enthalpy change of combustion - ΔHCθ. Enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions. Calculating Enthalpy of Reaction The value of enthalpy changes accompanying chemical reactions can be determined by using a calorimeter. Calorimeters can calculate the enthalpy changes of: Dissolving Neutralisation between acids and bases Formation Combustion CHEM 1 The heat energy change for any reaction can be calculated using: Heat (q) = mass of substance in KG (m) x specific heat capacity (c) x temperature change (ΔT) Q=mc ΔT Specific heat capacity is the amount of energy required to raise 1g -1 -1 of a substance by 1K. For pure water this is 4.18JK g . Enthalpy of solution – A known mass of solid is totally dissolved in a large excess of water whose mass is known. The change in temperature of the water is measured. Enthalpy of combustion – A known mass of a substance is combined. The heat energy released in the reaction is absorbed in a known volume of water and the temperature Energy Cycle Diagrams The law of conservation of energy – energy cannot be created or destroyed, only changed from one form to another. Hess’s Law – If a reaction can occur by more than one route, the overall enthalpy change is independent of the route taken. Bond Enthalpies Bond enthalpy – the standard enthalpy change associated with breaking one mole of bonds in a gaseous substance into individual gaseous atoms. The values are also positive because breaking bonds requires energy. The value for making a bond is the same, but with the opposite sign. Mean bond enthalpies – bond enthalpy values are dependent on the attraction between the nuclei of the atoms involved in the bond and the electrons shared between them. The enthalpy values will therefore be slightly different, depending on the environment of the particular CHEM 1 atom. The data quoted for enthalpy calculations is always a mean bond enthalpy. Breaking covalent bonds – endothermic Making covalent bonds – exothermic The standard enthalpy change for a reaction is calculated by: Calculating the energy needed to break all the bonds in the reactant molecules Calculating the energy released when all the bonds in the product molecules are made Subtracting the energy released in making bonds from the energy needed to break bonds