Inorganic Molecules
advertisement

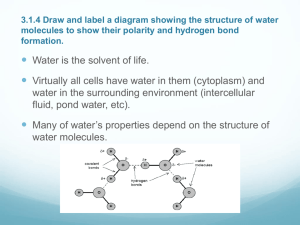
Inorganic Molecules Inorganic compounds are compounds that don’t contain C and H together. Inorganic compounds that are important for living organisms include water, oxygen, carbon dioxide, nitrogen and minerals. Oxygen • In most living organisms, oxygen is needed to release energy from food molecules. Organisms can get oxygen from breathing air or from water. • Organisms find it more difficult to get oxygen from water so they tend to be small, flat and relatively inactive or they are highly adapted with organs like gills to aid extraction. Carbon dioxide • Carbon is an extremely important part of all living things and needs to be cycled through ecosystems. Carbon dioxide is the main source of carbon for the production of the organic molecules from which living organisms are built. Carbon cycle Nitrogen • Nitrogen is a component of all proteins and is therefore required by organisms in large amounts. Nitrogen enters living things via the nitrogen cycle. The nitrogen cycle Minerals • Mineral salts are naturally, occurring inorganic compounds produced by the weathering of rocks. Important minerals required by organisms include phosphorus, potassium calcium, magnesium, iron, sodium, iodine and sulphur. Others are required in trace amounts. Plants absorb minerals through their roots, making them available to be eaten by animals. Phosphorus cycle Water • Water is the most abundant compound in our bodies. Water is the predominant solvent in living organisms ( this means that things can dissolve in it), it has a high heat capacity and it is highly cohesive. This is all due to its structure. Structure of water • Each water molecule consists of a combination of a single oxygen atom with two hydrogen atoms. Each hydrogen atom is linked to the oxygen atom by a strong covalent bond (created by atoms sharing electrons). • Although water has an overall neutral charge, the oxygen at the end of a covalent bond is slightly negative and the hydrogen atoms are slightly positive areas Animation • http://programs.northlandcollege.edu/biolo gy/Biology1111/animations/hydrogenbonds .html Cohesiveness • Individual molecules of water are highly attracted to each other such that the negative oxygen of one molecule of water is attracted to the positive hydrogen of another water molecule. These bonds that hold them together are called hydrogen bonds which are weaker than covalent bonds. This means that the hydrogen bonds that hold them together are relatively weak and are continually breaking and rejoining. As a solvent • Water is the predominant solvent in living organisms. Its versatility as a solvent is due to the cohesive nature of the molecule. • Substances that dissolve readily in water are called hydrophilic or polar. • Substances that tend to be insoluble in water are called hydrophobic or non-polar. • How do substances dissolve in water? As a solvent • http://programs.northlandcollege.edu/biolo gy/Biology1111/animations/dissolve.html Density • Most liquids contract (get smaller) when they get colder. Water is different. Water contracts until it reaches 4 C then it expands until it is solid. Solid water is less dense that liquid water because of this. If water worked like other liquids, then there would be no such thing as an ice berg, the ice in your soft drink would sink to the bottom of the glass, and ponds would freeze from the bottom up! Surface tension • Surface tension is the name we give to the cohesion of water molecules at the surface of a body of water. Try this: place a drop of water onto a piece of wax paper. Look closely at the drop. What shape is it? Why do you think it is this shape? • What is happening? Water is not attracted to wax paper (there is no adhesion between the drop and the wax paper). Each molecule in the water drop is attracted to the other water molecules in the drop. This causes the water to pull itself into a shape with the smallest amount of surface area, a bead (sphere). All the water molecules on the surface of the bead are 'holding' each other together or creating surface tension. Capillary action • Surface tension is related to the cohesive properties of water. Capillary action however, is related to the adhesive properties of water. You can see capillary action 'in action' by placing a straw into a glass of water. • The water 'climbs' up the straw. What is happening is that the water molecules are attracted to the straw molecules. When one water molecule moves closer to a the straw molecules the other water molecules (which are cohesively attracted to that water molecule) also move up into the straw. Capillary action is limited by gravity and the size of the straw. • Plants take advantage of capillary action to pull water from the soil into themselves. From the roots water is drawn through the plant by another force, transpiration. Water resists changes in temperature. • Hydrogen bonding is the cause, like for so many of water's properties, for it's high specific heat capacity. Heat is released when hydrogen bonds form and heat must be absorbed in order to break hydrogen bonds. • A calorie of heat causes a rather tiny change in the temperature because most of the heat energy is used to disrupt hydrogen bonds before the molecules can begin moving faster. As the temperature of water drops, many additional hydrogen bonds form at the same time. This releases a substantial amount of energy in the form of heat. Summary of properties Properties Chemical Reason Effect Resists changes in temperature Hydrogen bonding Helps keep body temperature constant Universal solvent Polarity Facilitate chemical reactions Is cohesive and adhesive Hydrogen bonding: polarity Serves as a transport medium. Capillary effect Has a high surface tension Hydrogen bonding Difficult to break surface tension Less dense as ice than as liquid water Hydrogen bonding Ice floats on water