BB30055: Genes and genomes
advertisement
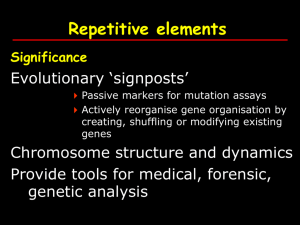
BB30055: Genes and genomes Genomes - Dr. MV Hejmadi (bssmvh@bath.ac.uk) Lecture 2 – Repeat elements Repetitive elements Significance Evolutionary ‘signposts’ Passive markers for mutation assays Actively reorganise gene organisation by creating, shuffling or modifying existing genes Chromosome structure and dynamics Provide tools for medical, forensic, genetic analysis Repetitive elements Main classes based on origin Tandem repeats Interspersed repeats Segmental duplications 1) Tandem repeats Blocks of tandem repeats at subtelomeres pericentromeres Short arms of acrocentric chromosomes Ribosomal gene clusters Tandem / clustered repeats Broadly divided into 4 types based on size class Size of repeat Repeat block Major chromosomal location Satellite 5-171 bp > 100kb centromeric heterochromatin minisatellite 9-64 bp 0.1–20kb Telomeres microsatellites 1-13 bp < 150 bp Dispersed HMG3 by Strachan and Read pp 265-268 Satellites Large arrays of repeats Some examples Satellite 1,2 & 3 a (Alphoid DNA) - found in all chromosomes b satellite HMG3 by Strachan and Read pp 265-268 Minisatellites Moderate sized arrays of repeats Some examples Hypervariable minisatellite DNA - core of GGGCAGGAXG - found in telomeric regions - used in original DNA fingerprinting technique by Alec Jeffreys HMG3 by Strachan and Read pp 265-268 Microsatellites VNTRs - Variable Number of Tandem Repeats, SSR - Simple Sequence Repeats 1-13 bp repeats e.g. (A)n ; (AC)n 2% of genome (dinucleotides - 0.5%) Used as genetic markers (especially for disease mapping) Individual genotype HMG3 by Strachan and Read pp 265-268 Microsatellite genotyping design PCR primers unique to one locus in the genome .a single pair of PCR primers will produce different sized products for each of the different length microsatellites How are tandem repeats generated in the genome? strand slippage during replication Fig 11.5 HMG3 by Strachan and Read pp 330 strand slippage during replication Fig 11.5 HMG3 by Strachan and Read pp 330 2) Interspersed repeats A.k.a. Transposon-derived repeats ~ 45% of genome Arise mainly as a result of transposition either through a DNA or a RNA intermediate Interspersed repeats (transposon-derived) major types class size Copy % number genome* LINE L1 (Kpn family) L2 ~6.4kb 0.5x106 0.3 x 106 16.9 3.2 SINE Alu ~0.3kb 1.1x106 10.6 LTR e.g.HERV ~1.3kb 0.3x106 8.3 mariner ~0.25kb 1-2x104 2.8 DNA transposon family * Updated from HGP publications HMG3 by Strachan & Read pp268-272 LINEs (long interspersed elements) Most ancient of eukaryotic genomes Autonomous transposition (reverse trancriptase) ~6-8kb long, located mainly in euchromatin Internal polymerase II promoter and 2 ORFs 3 related LINE families in humans – LINE-1, LINE-2, LINE-3. LINE-1 still active (~17% of human genme) Believed to be responsible for retrotransposition of SINEs and creation of processed pseudogenes LINEs (long interspersed elements) Nature (2001) pp879-880 HMG3 by Strachan & Read pp268-272 SINEs (short interspersed elements) Non-autonomous (successful freeloaders! ‘borrow’ RT from other sources such as LINEs) ~100-300bp long Internal polymerase III promoter No proteins Share 3’ ends with LINEs 3 related SINE families in humans – active Alu, inactive MIR and Ther2/MIR3. 100-300bp 1,500,000 13% Alu repeats evolved from processed copies of the 7SL RNA gene LINES and SINEs have preferred insertion sites • In this example, yellow represents the distribution of mys (a type of LINE) over a mouse genome where chromosomes are orange. There are more mys inserted in the sex (X) chromosomes. Try the link below to do an online experiment which shows how an Alu insertion polymorphism has been used as a tool to reconstruct the human lineage http://www.geneticorigins.org/geneticorigins/ pv92/intro.html Long Terminal Repeats (LTR) Repeats on the same orientation on both sides of element e.g. ATATATnnnnnnnnnnnnnnATATAT • contain sequences that serve as transcription promoters as well as terminators. • These sequences allow the element to code for an mRNA molecule that is processed and polyadenylated. • At least two genes coded within the element to supply essential activities for the retrotransposition mechanism. • The RNA contains a specific primer binding site (PBS) for initiating reverse transcription. • A hallmark of almost all mobile elements is that they form small direct repeats formed at the site of integration. Long Terminal Repeats (LTR) Autonomous or non-autonomous Autonomous LTR encode retroviral genes gag, pol genes e.g HERV Non-autonomous elements lack the pol and sometimes the gag genes e.g. MaLR Nature (2001) pp879-880 HMG3 by Strachan & Read pp268-272 DNA transposons (lateral transfer?) DNA transposons Inverted repeats on both sides of element e.g. ATGCNNNNNNNNNNNCGTA Nature (2001) pp879-880 From GenesVII by Levin 3) Segmental duplications Closely related sequence blocks at different genomic loci Transfer of 1-200kb blocks of genomic sequence Segmental duplications can occur on homologous chromosomes (intrachromosomal) or non homologous chromosomes (interchromosomal) Not always tandemly arranged Relatively recent Segmental duplications Interchromosomal segments duplicated among non homologous chromosomes Prone to deletions/ duplications Nature Reviews Genetics 2, 791-800 (2001); Intrachromosomal duplications occur within a chromosome / arm Prone to translocations Segmental duplications in chromosome 22 Segmental duplications Segmental duplications - chromosome 7. Pathogenic potential of Short Tandem Repeats (STR) Reduction or expansion of STR can be pathogenic 1) Unstable expansion of short tandem repeats Characterised by anticipation Large expansions outside coding sequences Modest expansions within coding sequences FRAXA, FRAX E Huntington disease (HD) Myotonic dystrophy (DM1) SCA 1,2,3,6,7, 17 Friedrich ataxia (FA) Kennedy disease Spinocerebellar ataxia 8,11 Unstable deletions of STRs? STRs tend to be deletion hotspots Interspersed repeats are susceptible to deletions/duplications E.g. Kearns-Sayre syndrome- encephalomyopathy External opthalmoplegia Ptosis Ataxia Common 4977bp deletion in mt DNA Cataract Pathogenic potential of segmental duplications Nature Reviews Genetics 2, 791-800 (2001) References 1) Chapter 9 pp 265-268 HMG 3 by Strachan and Read 2) Chapter 10: pp 339-348 Genetics from genes to genomes by Hartwell et al (2/e) 3) Nature (2001) 409: pp 879-891