Chemical reactions
advertisement

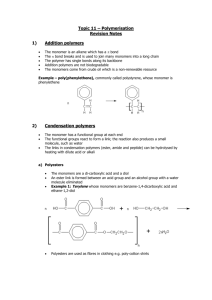
HAPPY MONDAY BUFFS!! Bellwork: (Have out your HW) Anticipation Guide: Write down “Agree” or “Disagree” for each statement. I love to bake cakes. 2. If you leave sugar out of the cake it, it will taste the same. 3. I like to eat cookies with milk. 4. If you mix glue and water, it will make a liquid. 5. A polygon has more than one side. 1. CO: I will understand how Polymers are made. LO: I will write notes on Monomers and Polymers and participate in the “Gloop Lab”. BIOMOLECULES (A QUICK RECAP) BIO = LIFE MOLECULE 4 major biomolecules: 1. 2. 3. 4. = ATOMS BONDED TOGETHER Carbohydrates Lipids Proteins Nucleic acids (we will talk about this one later) Biomolecules are molecules that are essential for life We find biomolecules in our FOOD! TURN TO PAGE Title: 18 OF YOUR I.A.N. Gloop Lab and Polymers Essential Question: How are large molecules formed? Set up Cornell Notes. The underlined words will need to go on the left. BIOCHEMISTRY: THE CHEMISTRY OF LIFE Matter: anything that takes up space Atom: basic unit of matter, building block of a molecule Molecule: two or more atoms bonded together CHEMICAL REACTIONS Chemical reactions are how ATOMS bond together and break apart. PARTS OF A CHEMICAL REACTION Arrow: “yields”, makes Reactants: what goes INTO the chemical reaction, left side of arrow Products: what is produced from the reaction, right side of arrow Chemical Reactions in a Cake MONOMER Mono = one Monomers are small molecular units that can be combined to make larger units. Monomers are the subunits of polymers POLYMERS Poly = many Polymer = a large molecule made by bonding smaller molecular units (monomers) together Monomer + monomer + monomer +…= polymer What is the monomer in the above polymers pictured? EXAMPLES OF BIOLOGICAL POLYMERS INCLUDE: Proteins, Complex Carbohydrates, Lipids, Nucleic Acids (DNA) Dehydration synthesis = a process through which polymers are formed from monomers through the removal of H2O. Hydro=water Lysis=cut Hydrolysis = the process of breaking down polymers into monomers by the addition of H2O. Hint: Think red rover DIRECTIONS FOR MAKING “GLOOP” *DECIDE ON ONE “MATERIALS PERSON” PER TABLE, THIS IS THE ONLY PERSON ALLOWED TO LEAVE THE LAB GROUP* 1. 2. 3. 4. In your plastic baggie, add glue up to the 1 cm line (Use a ruler: CM not Inches). Add 2-4 drops of food coloring to the glue and knead together with your hands. Add 7mL of water to the baggie using the marked graduated cylinder and mix thoroughly. Add 8 mL of borax solution to the baggie using the syringe and mix thoroughly. (Mix the solution in the beaker before using.) Gloop Lab Report: Copy what is in bold and answer the questions: 1. When we added water, what process was occurring: dehydration synthesis or hydrolysis? Describe the glue after water was added. 2. When we added borax, what process was occurring: dehydration synthesis or hydrolysis? Describe the glue after borax was added. 3. Summarize what happened during the Gloop Lab in 30 words. You must use the words: polymer, hydrolysis, dehydration synthesis