Chapter 5
advertisement

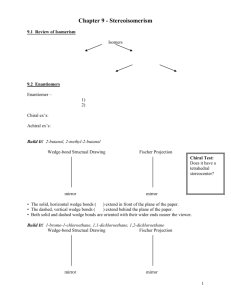
Stereochemistry Chiral Molecules Stereochemistry Stereosiomers are molecules Same formula and connectivity Differ by arrangement in space only Molecules are non-superimposable Not identical Achiral and Chiral Carbon Tetracarbon with 4 different atoms/groups attached chrial center or chiral carbon Test for Chirality: Planes of Symmetry mirror plane: an imaginary plane that bisects a molecule in such a way that two halves of the molecule are mirror images of each other Molecules with plan of symmetry are achiral Chiral objects Chiral Molecules Has no plane of symmetry Have non-superimposable mirror image Chiral Molecules Molecules that have no plane of symmetry Molecules that have non-superimposable mirror image Your left hand and right hand Subdivision of Isomers Stereoisomers Enantiomers: stereoisomers whose molecules are nonsuperimposable mirror image of each other Exact MW, BP, MP ect …except how it rotates in polarized light Stereoisomer Diastereoisomers: stereoisomers whose molecules are not mirror image of each other Nomenclature of Enantiomers: The R,S-System Each chiral carbon in a chiral molecule is designated a “R” (Rectus: right) or “S” (Sinitrus: left) configuration Rules: Assign priorities to atoms directly attached to chiral carbon based on Atomic number # 1 = highest atomic # # 4 = lowest atomic # Nomenclature of Enantiomers: The R,S-System If there’s a tie (e.g two carbons) move to the next carbon atom until tie is broken If there’s a double bond = 2 single bonds With the lowest priority group (#4) point away from you, rotate 14 Clockwise R configuration Counterclockwise S configuration Nomenclature of Enantiomers: The R,S-System Vinyl group, isopropyl group is of higher priority than the Examples Assign (R) or (S) designation to each of the following compounds Molecules with Multiples Chiral Centers The maximum number of stereiosomers can be predicted 2n n = number of chirality centers Naming compounds with multiple chirality center Followed same rules for naming R,S Designate position of chiral carbon Molecules with multiple chirality center Examples Draw alll possible stereoisomers and provide an appropriate name for each stereoisomer Fisher Projection Formulas Show three dimension structure Compound with several chiral centers Used carelessly, these projection formulas can easily lead to incorrect conclusion Examples Draw all stereoisomers of Meso compounds A structure with two chirality centers does not always have four possible stereoisomers. achiral occurred within chiral molecules when carbon is inverted Look for internal plane of symmetry Properties of Enantiomers Recall: the molecules of enatiomers are not superposable one on the other Enantiomers have identical M.P, B.P Different physical properties and direction which they rotate plane-polarized light Same amount in rotation but opposition direction Different reaction rate when interact with another chiral moleucle Plane-Polarized light When regular light beam is passed through a polarizer, all of the light waves, except those whose electromagnetic fields ossicllate in a single direction, are filter out plane-polarized light optically active Plane-Polarized light Polarimeter A substance that is rotated in a clockwise rotation α (measured of degree) is positive (+) Dextrorotatory A substance that is rotated in a counterclockwise rotation α is negative (-) levorotatory Specific Rotation Specific Rotation Depends on the temperature and the wavelength of light that is employed Specific Rotation The direction of rotation of plane-polarized light is often incorporated into the names of optically active compounds No obvious correlation exists between the (R) and (S) configurations of enantiomers and the direction ([(+) or (-)] in which they rotate plane-polarized light Racemic Mixtures An equimolar mixture of two enantiomers is called a racemic mixture (racemate, racemic form) No net rotation of plane-polarized light 50:50 mixture Resulted from chemical reaction of achiral molecule Enantiomeric excess A sample of an optically active substance that consists of a single enantiomers is said to be enantiomerically pure or to have an enantiomeric excess of 100% Resolution: Separating Enantiomers A common way of separation uses the conversion into diastereomers, that are not mirror images of each other Recrystallization or chromatography Resolution: Separating Enantiomers Stereoisomerism of cyclic compounds Is trans-1,2-dimethylcyclopentane superposable on its mirror image Is cis-1,2-dimethylcyclopentane superposable on its mirror image? Is cis-1,2-dimethylcyclopentane a chiral molecule? Would cis-1,2-dimethylcylcopentane show optical activity? What is the stereoisomeric relationship between 1 and 3 Cyclohexane Derivative Compounds with Chirality Centers Other than Carbons