Chapter four " reaction kinetics" chemical reactions.
advertisement

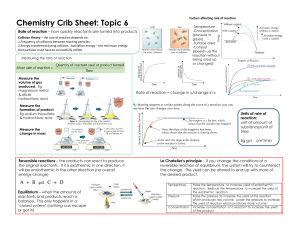
Chapter four " reaction kinetics" Chemical kinetics: is the branch of physical chemistry that deals with a study of the speed of chemical reactions. There are two types of reactions: Homogeneous reactions: take phase entirely within one phase such as reactions between gas molecules to produce gas product. Heterogeneous reactions:involve mor than one phase such as the reaction between gaseous oxygen and a solid metal to form an oxide film on the metal surface. Rate of reaction : is the velocity of a reaction ,is the amount of chemical change occurring per unit time. It is generally defined as the decrease in the concentration of the reactant or an increase in the concentration of the products per unit time. Factors influencing the reaction rate : nature of reactants and products,effect of temperature, concentration of reactants, effect of catalyst ,surface area of reactants, radiation. Rate constant : consider the following reaction RATE of reaction=r= k=proportionality constant called the velocity constant,rate constant,or specific reaction rate at a given temperature. In the life of a chemical reaction the concentration and the rate are ever changing .the specific rate constant is the only constant factor directly related to the mechanism of the reaction