Chemistry Exam Review 2016 Answers
advertisement

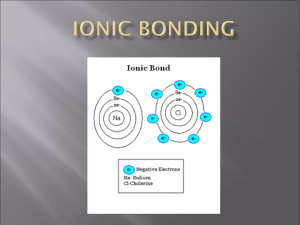
Chemistry Exam Review Answers: Have you tried the self quizzes on pg. 204, 250, 282, and Unit Review pg. 288-293 Q#1-10,13-25,26-39,40-41,43-46,49,51,52,55,63 SELF TEST Unit C pg. 294-295 1. What is the density of a liquid that has a mass of 5.5 g and takes a volume of 11.0 mL? m = 5.5 g v = 11.0 L D=? D = m/v D = 5.5 g / 11.0 L D = 0.5 g/mL Therefore the density of the liquid is 0.5 g/mL 2. i. A block of wood measures 3 cm by 6cm by 2 cm. It has a mass of 10 g. Calculate the density of the block of wood. v=? m = 10 g L = 3 cm, W = 6 cm, H = 2 cm D=? v=LXWXH v = 3 cm X 6 cm X 2 cm v = 36 cm3 D = m/ v D = 10 g / 36 cm3 D = 0.28 g/cm3 Therefore the density of the block of wood is 0.28 g/cm3 ii. There is a graduated cylinder that contains 20 mL of water. You add 250 g of lead weights and the volume of water rises to 42 mL. What is the density of the lead weights? M = 250 g v1 = 20 mL v2 = 42 mL v=? D=? v = v2 – v1 v = 22 mL D = m/v D = 250 g / 22 mL D = 11.4 g / mL Therefore the density of the lead weights is 11.4 g/mL 3. How could you measure the density of a) a solid object (i.e. plasticine) with an irregular shape? b) a liquid a) The density of a solid with an irregular shape is measured through the displacement of water technique. This involves first massing the irregular solid. Then water is added to a graduated cylinder and measured. Finally, the object is submerged into the water and the final volume measured. The volume of the solid is obtained by subtracting the initial volume from the final volume. Density is then calculated by dividing the mass by the volume. b) The density of a liquid is obtained by first measuring the mass of a clean, dry graduated cylinder. Add an amount of liquid to the cylinder and record both the new mass with the liquid and the volume of liquid added. The mass of the liquid is obtained by subtracting the mass of the cylinder from the total mass of cylinder and liquid. Finally calculate density by dividing this mass by the liquid’s volume. 4. Identify a) the total number of atoms, and b) the total number of H atoms, in each of the following: i. CH3COOH 2 carbon atoms, 4 hydrogen atoms and 2 oxygen atoms ii. H2SO4 2 hydrogen atoms, 1 sulfur atom and 4 oxygen atoms 6. Complete the following table: Use a periodic table to help you. Symbol Atomic Number Mass number p+ no e- overall charge 231Ra 88 88 231 88 143 88 0 107Ag 47 107 47 60 47 0 9F 9 18 9 9 10 -1 211Cm 96 211 96 115 93 +3 92 240 92 148 86 +6 47 18 96 240U 92 7. i. Draw the Bohr diagrams of the following and the Bohr diagram for the most likely ion formed. 24 a. 12 b. Chlorine c. Neon (How many valence electrons does neon have?) Mg ii. Using Lewis dot diagrams ,illustrate what happens to the valence electrons when sodium and chlorine bond together to form an ionic compound (before, during, after) include the name of the compound and the chemical formula of the substance formed. 8. Use the periodic table to locate and give an example of an element in each of the following groups of elements located on the periodic table? a. Metals lithium, sodium, iron, magnesium, calcium, gold, silver, copper (any element left of the metalloid staircase) b. Non-metals staircase) nitrogen, oxygen, fluorine, chlorine (any element to the right of the metalloid c. Metalloids boron, silicon, germanium, arsenic, antimony, tellurium (must be touching the staircase with the exception of aluminum) d. Halogens fluorine, chlorine, bromine, iodine (group 17 or 7) e. Noble gases helium, neon, argon, krypton, xenon (group 18 or 8) f. Alkaline Earth Metals g. Alkali metals beryllium, magnesium, calcium, strontium (group 2) lithium, sodium, potassium rubidium (group 1) 9. i. What is the same about the electron arrangement of all halogen elements? All halogen elements are found in group 7 and thus have 7 valence or outer shell electrons. ii. What group of elements are they likely to react with: Noble gases, alkali metals, alkaline earth metals, or other halogens? The halogens are most likely to react with the alkali metals. This is the easiest interaction as the alkali metals want to give up one electron and the halogens only want to gain one electron. The halogens will not react with Noble gases as the Noble gases have a stable (full) outer valence shell. The halogens can also react with the alkaline earth metals but not in a one to one ratio. The alkali metals can also form covalent or sharing electron bonds with other halogens. 10. Give the general formula of an ionic compound formed from a group II element (X) and a group VII element (Y). Group II elements have 2 valence electrons and thus lose these electrons to form stable X2+ ions. Group VII elements have 7 valence electrons and thus gain one electron to form stable Y1- ions. When these two groups interact, the positive ion will need two negative ions to receive its 2 electrons. Thus the formula will be XY2 11. Name or give the formula for the following ionic compounds: a. NaI sodium iodide b. magnesium fluoride MgF2 c. CaCl2 calcium chloride d. aluminum sulfide Al2S3 e. KI potassium iodide f. beryllium nitride Be3N2 g. ZnO zinc oxide h. lithium oxide Li2O 12. What is the difference between the two isotopes of carbon: carbon-14 and carbon-12? Carbon-14 has two more neutrons than carbon-12. 13. You can use a Venn diagram to help compare (similarity/difference/examples) the following sets of terms: a. mass # and atomic # c. malleability and ductility e. clarity and luster g. physical and chemical change b. metal and non-metal d. mixture and pure substance f. physical and chemical property h. qualitative and quantitative property i. reactants and products j. atom and ion k. element and compound and molecule 14. State the test and positive result for oxygen gas, hydrogen gas and carbon dioxide gas. 15. Use the following chemical equation to answer the questions below 2Mg ( s ) O2( g ) 2MgO( s ) a. Identify a reactant and its state There are two reactants solid magnesium and gaseous oxygen. b. What is the name of the product? The product is called magnesium oxide. c. If you burned 3.0 g of magnesium and produced 5.0 g of product, how much oxygen gas was used? The law of conservation of mass states that matter cannot be created or destroyed. Thus for 5.0 g of product to be made there must have been 2.0 g of oxygen combined with the 3.0 g of magnesium. d. Is this a chemical or physical reaction? This is a chemical reaction as a new substance was created. 16. List 5 clues that indicate a chemical reaction has taken place. Bubbling or a new smell A dramatic colour change The formation of a precipitate (solid from two liquids) The release or absorption of energy Production of brand new substances in any state