C5 revision PowerPoint - Blackpool Aspire Academy
advertisement

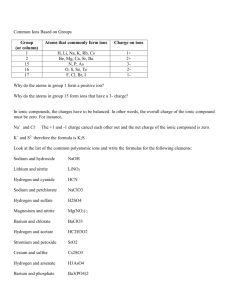
C5 Chemical of the natural environment What is the atmosphere? Dry air is a mixture of gases. Our atmosphere is made up of the following: 78% Nitrogen 21% Oxygen 1% Argon 0.04% Carbon Dioxide Chemicals of air consists of atoms and small molecules. There are weak forces of attraction between the molecules so these chemicals have low melting and low boiling points. Most non-metals elements and compounds of non-metals are molecular so they have low melting and boiling points. The atoms in a molecule are joined together by strong covalent bonds – one or more pairs of electrons shared between atoms. Covalent bonding arises from electrostatic forces of attraction between the shared electrons and the nuclei of the atoms. Pure molecular compounds cannot conduct electricity because their molecules are not charged. What are ionic compounds? Seawater is a mixture of water and dissolved ionic compounds called salts. Ionic compounds are made up of positive and negative ions. In ionic solids, the ions are arranged in a giant three dimensional lattice to make crystals. There are very strong attractive forces between the oppositely charges ions. This is ionic bounding. Ionic compounds have high melting and boiling points because much energy is needed to break an ionic structure. A solid ionic compounds cannot conduct electricity but when ionic compound melts or dissolves in water, its ions are free to more independently. It can now conduct electricity. How can we identify ions? There are simple tests to identify ions and work because each ion and each compound has its own properties. If you mix certain solutions of ionic compounds, you make an insoluble compound forming a precipitate. E.g. + Adding an alkali to a solution that contains Cu ions make a blue precipitate of Cu(OH)2 CuCl2 (aq) + 2NaOH(aq) 2NaCl(aq) + Cu(OH)2(s) Solutions containing some other metal ions also react with alkalis in solution to make precipitates. Adding acidified a silver nitrate solution to a solution of Clions makes a white precipitate of silver chloride AgNO3(aq) + NaCl(aq) AgCl(s) +NaNO3(aq) How can we identify ions? You can use solubility data to predict chemicals that will precipitate on mixing solutions. A compound with a low solubility will form as a precipitate and if both the products are soluble no precipitate will form. What are giant structures? The lithosphere is the Earth’s rigid outer layer and consists of part of mantle and crust. It is a mixture of minerals – compounds that occur naturally. Silicon , oxygen and aluminium are the most common elements in the Earth’s crust and much of the silicon and oxygen exists as a compound, silicon dioxide – solid silicon dioxide has a giant structure of atoms, which are held together in a huge lattice by strong covalent bonds. In diamond and graphite are also minerals which both consists of carbon atoms arranged in giant structures. What are giant structures? In diamond, each carbon atom is joined to four other carbon atoms by strong covalent bonds and the four bonds are arranged in three dimensions around each atom. In graphite, each carbon atoms is joined to three other carbon atoms by strong covalent bonds and the three bonds are arranged around each carbon atoms, making sheets of hexagons and between the sheets, or layers are free electrons which help to stick the layers together. Diamond Graphite What are giant structures? Graphite Diamond Silicon Dioxide Melting and boiling points Very high because much energy is needed to break the strong covalent bonds in the giant structure of atoms Solubility in water Insoluble because much energy is need to break the strong covalent bond Hardness Soft because the forces between the layers are weak. A good lubricant/ Very hard because much energy is need to break the strong covalent bonds between the surface of atoms. Diamond is useful for tools and drill tips Electrical conductivity Good because the electrons between the layers are free to move Do not conduct electricity because no charge particles are free to move. Why are metals useful? Metals have many uses and their uses depend on their properties. Metals have high melting points and are: Malleable Strong Good electrical conductor Solid metals have giant crystalline structures so strong metallic bonds hold the atoms together explaining why metals are strong and why they have high melting points. A metal crystal is made up of positive metal ions are arranged in layers. When you bend a metal the layers of ions slide over each other. The ions are held together by a sea of electrons that are free to move. When a metal wire conducts electricity, electrons drift from one end towards the other. How are metals extracted? In the lithosphere, most metals are joined to other elements in minerals. Rocks that contain useful minerals called ores. Copper is extracted from the mineral copper iron sulfide and ore that contains this mineral is copper pyrites. Ores contain different amounts of minerals and often, a huge amount of ore contains only a tiny mass of a useful mineral. How are metals extracted? Extracting metals by heating with carbon Iron, Copper, and Zinc are extracted from their oxides by heating with carbon. ZnO(s) + C(s) Zn(s) + CO(g) How are metals extracted? Extracting metals by electrolysis Reactive metal like Al2 are joined very strongly to other elements in the minerals and cannot be extracted by heating with carbon so they are extracted by electrolysis. Al2O3 is an ionic compound and when it melts, its ions can move independently. So liquid Al2O3 conducts electricity and is an electrolyte. Electrolytes decompose when an electric current passes through them. This is electrolysis. How are metals extracted? Extracting metals by electrolysis To extract aluminium from aluminium oxide: Melt aluminium oxide Pass an electrical signal through the electrolyte. Aluminium forms at the negative electrode. Al3+ ions gain electrons from the electrode to make neutral aluminium atoms. Al3+ + 3e- Al Oxygen forms at the positive electrode. O2- ions give electrons to the positive electrode to make oxygen atoms. O2- O + 2eO + O O2