Slide 1

NSAIDS – Aspirin, Ibuprofen,
Celebrex and Beyond
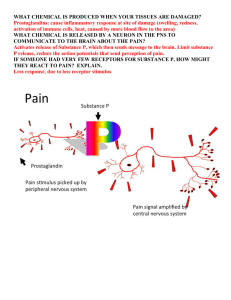
Prostaglandins
Prostaglandins have several differenct biological functions, one of which is to stimulate inflammation. The biological synthesis of prostaglandins includes several steps, starting with arachidonic acid: arachidonic acid prostaglandin synthase
PGH
2
(a peroxide) prostaglandin
O
O
COOH
CH
3
Aspirin, or O-acetyl-salicylic acid, has two carbonyl functional groups.
Which of those is more electrophilic or “activated”?
Prostaglandin synthase is composed of two enzymes. One of the enzymes, CycloOXygenase (COX), has a serine hydroxyl group that is necessary for enzyme activity. In the presence of aspirin, the hydroxyl group of cyclooxygenase becomes “acylated”. Aspirin is termed a non-reversible inhibitor, because it covalently modifies the active site of the enzyme and the enzyme is not functional while that covalent modification exists. Draw the electron pushing for that process.
Aspirin
That process is called “transesterification”. Note that the enzyme is now unable to catalyze the formation of prostaglandins. If prostaglandins are not synthesized, inflammation and associated pain does not occur.
aspirin
O
O
COOH
CH
3
COOH ibuprofen
Note that ibuprofen (Advil, Motrin, Nuprin) lacks the ester functional group present in aspirin. Since that ester functional group was involved in the chemistry between aspirin and the
COX enzyme, we might expect that ibuprofen has a different mode of action…
Ibuprofen
• Ibuprofen mimics arachadonic acid and fits into the active site of the
COX enzyme. While ibuprofen is in the active site, arachadonic acid cannot enter and prostagladin synthesis is inhibited. This is reversible inhibition.
Because of patent rights, legal issues and monetary considerations, scientists are constantly searching for new, more effective medications.
MeO
Naproxen (in Aleve)
COOH
Several different forms of the CycloOXygenase enzyme exist. COX-1
And COX-2 are the best understood. The structures of COX-1 and
COX-2 are similar, but these enzymes serve markedly different functions.
Arachidonic Acid
COX-2
“inflammatory” prostaglandins
COX-1 “housekeeping” prostaglandins
• platelets (for blood clotting)
• prostaglandin E2 (for kidney function)
• prostaglandin I2 (for stomach lining)
Scientists reasoned that if we could find compounds that are highly selective for the active site of COX-2, but are unable to bind to COX-1, we could manage pain/ inflammation without affecting other “housekeeping” functions.
COX-2 inhibitors
SO
2
CH
3 SO
2
NH
2
O
O
CF
3
N N
Vioxx Celebrex
These drugs were hailed as “super aspirins”… they are now off the general market because of negative side effects.
4 May 2000
Soon after the crystal structure in 2000, scientists proposed the structure of a molecule (APHS) which was calculated to fit perfectly into the active site, thus effectively shutting down the inflammatory/pain response.
O
O
S
• What mode of action would APHS be predicted to have?
• Is it wise to mask inflammation/pain?
• The mode of action of Tylenol is not yet understood ….