The Chemistry of Life: Organic and Biological Chemistry
advertisement
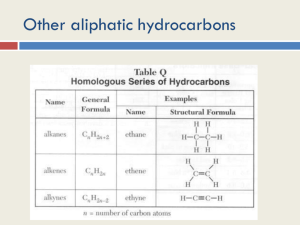
The Chemistry of Life: Organic and Biological Chemistry BLB 11th Chapter 25 25.1 General Characteristics Structure – Carbon atoms form 4 bonds. – Backbone consists of –C—C— chains or rings. – C—H bond is nearly nonpolar. Stabilities – C forms strong bonds with other elements. – Alkanes are stable (unreactive). – Functional groups (sect. 5) change reactivity. General Characteristics Solubility and acid-base properties – Hydrocarbons: nonpolar, neutral soluble in water? ______ – C—H (hydrocarbons) plus functional groups… -C—O—H polar, slightly acidic (alcohols) -C—O—O—H acidic (carboxylic acids) -C—N—R3 basic (amines) – Surfactants combine both properties 25.2 Hydrocarbons Compounds containing only C and H Types: – Alkanes (saturated) – single C–C bonds – Alkenes (unsaturated) – double C=C bonds – Alkynes (unsaturated) – triple C≡C bonds – Aromatic – alternating single and double bonds 25.3 Alkanes (CnH2n+2) Variations – Unbranched (normal, “straight-chained”) – Branched – Cyclic Structural isomers – same number and type of atoms but different bonding arrangements 3-D Arrangement Tetrahedral geometry Name Formula MM m.p. (°C) b.p. (°C) # isomers methane CH4 16 -183 -161 1 ethane C2H6 30 -183 -89 1 propane C3H8 44 -187 -44 1 butane C4H10 58 -138 -0.5 2 pentane C5H12 72 -130 36 3 hexane C6H14 86 -95 68 5 heptane C7H16 100 -91 98 9 octane C8H18 114 -57 125 18 nonane C9H20 128 -54 151 35 decane C10H22 142 -30 175 75 Butane Isomers Pentane Isomers Nomenclature 1. 2. 3. 4. Identify longest C chain → base name Number C atoms – lowest number nearest substituent Give position and name substituents. More than one substituent? Alphabetical Prefixes Some substituents have common names (Table 25.2, p. 1059) Structure & Nomenclature Examples Structure & Nomenclature Examples Structure & Nomenclature Examples Cycloalkanes (CnH2n) most stable stable structure structure strained & unstable Alkenes & Alkynes Alkenes: C=C double bonds – Named similarly with an –ene ending and give position of double bond – More reactive than alkanes – Different arrangements of substituents may result in geometric isomers. Geometric isomers – same atoms, same bonds, but different spatial arrangement of substituent groups. – cis- and trans- Structural isomers Geometric isomers Structure & Nomenclature Examples Structure & Nomenclature Examples Alkenes & Alkynes Alkynes: C C triple bonds – Named similarly with an –yne ending and give position of triple bond – More reactive than alkenes Aromatic Hydrocarbons Cyclic unsaturated hydrocarbons with alternating single and double C=C bonds. Ring system often has common name. Less reactive than alkenes or alkynes Bonding in Benzene 25.4 Organic Functional Groups Site of reactivity R, R', R" represent the alkyl (hydrocarbon) group(s) to which functional group is attached. Functional Group examples Aspartame Functional Group examples H H NH2 O N HO H CH2 CH CO2H 25.5 Chirality Chiral compounds – compounds containing carbon atoms with four different attached groups Br – Examples: CHFClBr, CH3—C—CH2CH2CH3 H Enantiomers – nonsuperimposable mirror images Enantiomers Enantiomers Chiral Compounds D- sugars are preferred L- amino acids are preferred Drugs – Ibuprofen: one effective, other inactive – Naproxen: one effective, other toxic