PPTX - CERN Teaching Materials
advertisement

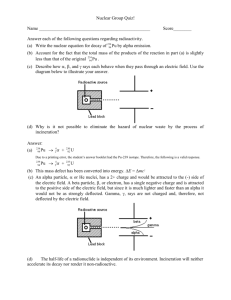
Teaching Resources HST 2008 A brief History of our thinking The Greek thinker Empedocles first classified the fundamental elements as fire, air, earth, and water, although our particular diagram reflects Aristotle's classification. Did you know? The ancient Chinese believed that the five basic components (in Pinyin, Wu Xing) of the physical universe were earth, wood, metal, fire, and water. And in India, the Samkhya-karikas by Ishvarakrsna (c. 3rd century AD) proclaims the five gross elements to be space, air, fire, water, and earth. People have long asked, "What is the world made of?" and "What holds it together?" As far back as in the days of Aristotle, it was thought that things were made up of four types of fundamental elements. The word "fundamental" is key here. By fundamental building blocks we mean objects that are simple and structureless -- not made of anything smaller. Based on scientific observations, our thinking has variously changed in the recent past. The story begins with John Dalton ...... Dalton suggested that … … everything is made up of very tiny particles. He named these smallest possible piece of an element ‘an atom’ – which in Greek means unbreakable. Further he suggested that…. •Atoms are different for different elements. •Imagine atoms to be solid like billiard balls. Oxygen Atom Hydrogen Atom Gold Atom From our scientific observations in chemical reactions, x-ray diffraction etc, we know today that this view was not entirely correct. Something was missing.. However, Dalton’s ideas became the basis for our modern quest for the real constituents of matter (and antimatter!) Possible web link to a CERN site with pictures, simulations or videos? Gold Atom Proposed after discovery of “cathode rays” when a gas is ionized by a high voltage. Neutral atoms contain smaller particles, called electrons. Electrons exist in a “sea of positive charge” like plums in a pudding. Shot high energy a particles at a thin gold foil Observed the pattern of scattered particles Found some scattered by a larger angle than predicted by the Thomson model Positive charge is concentrated in the nucleus (only way to produce large scattering angles observed) Coulomb force causes electrons to orbit the positive nucleus Planetary model Hydrogen Atom Accelerating electrons should radiate energy according to Maxwell’s Electromagnetic theory Radiating electrons are losing energy so they will spiral in towards the nucleus as they lose energy No atom would be stable -- all would radiate away their energy Predicted Hydrogen Emission Spectrum as atoms radiate energy Hydrogen electrons may only exist at certain distances from the nucleus (energy levels) If they stay in the same energy level, they are stable (don’t radiate) If they move from one level to another, they radiate to produce the discrete spectrum observed Ionized gas atoms are injected into a mass spectrometer All atoms have the same charge and the velocity selector guarantees that they had the same velocity Different radii are observed in the B-field deflection Only way for this is to have atoms with same charge and different mass. (R = mv/qB) There must be a neutral particle in the nucleus with significant mass = neutron Atoms with same charge (protons) and different mass (neutrons) are called Isotopes electron nucleus Helium Atom Specific Nucleus = nuclide Nuclear particles (protons and neutrons) = nucleons Identically charged nuclei with different mass = isotopes Generic nucleus symbol = Number of nucleons = A = mass number Number of protons = Z = charge (atomic) number A Z X Clearly, if the nucleus contains a number of protons, the Coulomb force would predict that the protons should repel each other There must some force that is stronger than the Coulomb force to hold them together = Strong Nuclear Force Strong Nuclear Force also keeps neutrons in check in the nucleus Acts like a spring between nucleons FE FE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle Alpha Particle KE charge = +Ze Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle charge = +2e charge = +Ze E = KE Alpha Particle KE Alpha Particle PE We can use the results from the scattering of positively charged particles to determine the nuclear radius a Particle charge = +2e charge = +Ze R E = PE = Alpha Particle KE Alpha Particle PE (2e)( Ze) 2Ze2 4 o R 4 o R We can use the results from the scattering of positively charged particles to determine the nuclear radius Initial E = (KE) Final E = PE = (2e)( Ze) 2Ze 2 4 o R 4 o R Conservation of Energy: (KE) = Solve for R = 2 Ze 2 4 o ( KE ) 2 Ze 2 4 o R We can use the results from the scattering of positively charged particles to determine the nuclear radius Solve for R = 2 Ze 2 4 o ( KE ) Adjust initial KE until the a particle is no longer scattered back. Last scattered KE gives estimate of R. Empirical Result: R = (1.2 x 10-15)A1/3 Alpha particles emitted by an unstable nucleus are found to have limited possible amounts of kinetic energy Gamma rays (pure energy) emitted by an excited nucleus are found to produce discrete spectra These results suggest that nuclei have energy levels just like atoms do The nature of the strong nuclear force is that it is effective over very small distances A-Z 12 0 10 0 80 Segre Plot of Stable Nuclides = stable 60 40 20 10 20 30 40 50 60 70 80 Z Small nuclei are stable when the number of neutrons = number of protons. The nature of the strong nuclear force is that it is effective over very small distances A-Z 12 0 10 0 80 Segre Plot of Stable Nuclides = stable 60 40 20 10 20 30 40 50 60 70 80 Z As Z increases, the number of neutrons necessary becomes larger than the number of protons. The nature of the strong nuclear force is that it is effective over very small distances A-Z 12 0 10 0 80 Segre Plot of Stable Nuclides = stable 60 40 20 10 20 30 40 50 60 70 80 Z For large values of Z, the number of neutrons becomes very large. The nature of the strong nuclear force is that it is effective over very small distances A-Z 12 0 10 0 80 Segre Plot of Stable Nuclides = stable 60 40 20 10 20 30 40 50 60 70 80 Z • For large nuclei, the Coulomb force wins out over the strong nuclear force The nature of the strong nuclear force is that it is effective over very small distances = range of nuclear force this proton can repel the proton on the opposite side of the nucleus this neutron is too far away to exert an attractive nuclear force on the nucleons on the opposite side of the nucleus • For large nuclei, the Coulomb force wins out over the strong nuclear force The nature of the strong nuclear force is that it is effective over very small distances = range of nuclear force this proton can repel the proton on the opposite side of the nucleus this neutron is too far away to exert an attractive nuclear force on the proton on the opposite side of the nucleus • In addition, as the nucleons become too tightly packed the nuclear force will cause them to repel each other. To achieve stability, the nucleus must either decrease its size by emitting clusters of nucleons (a particles) a particle emission To achieve stability, the nucleus must either decrease its size by emitting clusters of nucleons (a particles) a particle emission neutron decays into proton and b particle b particle emission • Or, it must rearrange the nucleons to balance the nuclear force and Coulomb force (b particles)