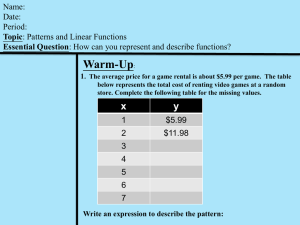
File
advertisement

CONCEPTUAL MAP INTRODUCTION Physical quantities Base quantities Prefixes Derived quantities Scientific notation (standard form Scalar quantities Vector quantities Dimensional Analysis * PHYSICAL QUANTITIES •A quantity that can be measured. •A physical quantities have numerical value and unit of measurement. •For example temperature 30 degrees celcius, 30 is numerical value & ‘degree celcius’ is the unit. Written as 30o C. Temperature Physical quantity = 30 degree Celcius = 30o c = numerical value x unit measurement * 1. A physical quantity is a quantity that can be measured and consists of a numerical magnitude and a unit. 2. The physical quantities can be classified into base quantities and derived quantities. 3. There are seven base quantities: length, mass, time, current, temperature, amount of substance and luminous intensity. 4. The SI units for length, mass and time are metre, kilogram and second respectively. 5. Prefixes are used to denote very big or very small numbers. BASE QUANTITIES •Base Quantities are physical quantities that cannot be derived from other physical quantities. •Scientific measurement using SI units (International System Units). Table 1.1 Shows five base quantities and their respective SI units Base Quantities Symbol SI Unit Symbol of SI unit Length L meter m Mass m kilogram kg Time t second s Temperature T Kelvin K Electric current I ampere A Chapter 1 Physical Quantities, Units and Measurement Scalars and Vectors • Vector quantities are quantities that have both magnitude and direction A Force Magnitude = 100 N Direction = Left THEME ONE: MEASUREMENT Chapter 1 Physical Quantities, Units and Measurement • Scalar quantities are quantities that have magnitude only. Two examples are shown below: Measuring Mass THEME ONE: MEASUREMENT Measuring Temperature Mass: The amount of matter in a body. • SI Units: kilogram (kg) • Common Units: pounds (lbs) and ounces (oz) 1 kg is approx. 2.2 lbs 1 kg = 1000 g 1 oz = 28.35 g This Platinum Iridium cylinder is the standard kilogram. 7 Length: A measure of distance. • SI Unit: meter (m) • Common Units: inches (in); miles (mi) 1 in = 2.54 cm = 0.0254 m 1 mi = 1.609 km = 1609 m 8 Volume: Amount of space occupied by a body. • SI Unit: cubic meter (m3) • Common Units: Liter (L) or milliliter (mL) or cubic centimeter (cm3) 9 Density: Amount of mass per unit volume of a substance. • SI Units: kg/m3 • Common Units: g/cm3 or g/mL 10 Measuring Systems International system SI Unit (m K s) International system (c g s) Unit International system (f b s) Unit 1.2 SI Units • Example of derived quantity: area Defining equation: area = length × width L In terms of units: Units of area = m × m = m2 Defining equation: W volume = length × width × height In terms of units: Units of volume = m × m × m = m3 Defining equation: H density = mass ÷ volume In terms of units: Units of density = kg / m3 = kg m−3 L W 1.2 SI Units • Work out the derived quantities for: Defining equation: velocity = displaceme nt time In terms of units: Units of speed = m/s Defining equation: acceleration = velocity time In terms of units: Units of acceleration = m/s2 Defining equation: force = mass × acceleration In terms of units: Units of force = Kg m/s2 1.2 SI Units Defining equation: Work = Force x Displacement In terms of units: Units of Work = J = Kg m2/s2 Defining equation: Energy = Mass x gravity x high In terms of units: Units of Energy = J = Kg m2/s2 Defining equation: Power = Force x displacement / time In terms of units: Units of Power = W = Kg m2/s3 Defining equation: Pressure = Force / area In terms of units: Units of Pressure = Kg m /m2 s2 = Kg m-1/s2 = N/m2 DERIVED QUANTITIIES • Derived Quantities are physical quantities derived from combination of base quantities through multiplication or division or both Table 1.2 shows some of the derived quantities and their respective derived units Derived Quantities Symbol Relationship with base quantities Derived units Area A Length x Length m2 Volume V Length x Length x Length m3 Density ρ Mass Length x Length x Length kg/m3 Velocity v Displacement Time m/s Acceleration a Velocity Time m/s2 Force F Mass x Acceleration N Work W Force x Displacement J Energy Ep Ek Mass x gravity x high = ½ x mass x velocity x velocity J Power P Force x Displacement Time W Pressure p Force Area N/m 2 Temperature: is the measure of how hot or cold an object is. • SI Unit: Kelvin (K) • Common Units: Celsius (ºC) or Fahrenheit (ºF) Converting between K , ºC and ºF: TK= TC+273.15 TC= TK – 273.15 TF= 9/5 TC + 32 TC= 5/9 (TF – 32) 16 Black board example 19.1 “When the thermometer is held in the mouth or under the armpit of a living man in good health” it indicates 98 F a) What is the temperature in Celsius (centigrade)? b) What is the temperature in Kelvin? PREFIXES Prefix • • Prefixes : are used to simplify the description of physical quantities that are either very big or very small. Table 1.4 Lists some commonly used SI prefixes Peta tera giga mega kilo hekto deka deci centi milli micro nano pico Femto Symbo l P T G M k h da d c m m n P F Value 1015 1012 109 106 103 102 10 10-1 10-2 10-3 10-6 10-9 10-12 10-15 STANDARD FORM Standard form or scientific notation is used to express magnitude in a simpler way. In scientific notation, a numerical magnitude can be written as : A x 10n, where 1 ≤ A < 10 and n is an integer Example 1.1 : I. II. For each of the following, express the magnitude using a scientific notation. The mean radius of the balloon = 100 mm The mass of a butterfly = 0.0004 kg Solution: The mean radius of the balloon = 100 mm =100 x 10-3 m = 0.1 m The mass of a butterfly = 0.0004 kg =0. 0004 x 103 g = 0.4 g * Dimensional Analysis The word dimension in physics indicates the physical nature of the quantity. For example the distance has a dimension of length, and the speed has a dimension of length/time. The dimensional analysis is used to check the formula, since the dimension of the left hand side and the right hand side of the formula must be the same. Example Using the dimensional analysis check that this equation x = ½ at2 is correct, where x is the distance, a is the acceleration and t is the time. Solution x = ½ at2 This equation is correct because the dimension of the left and right side of the equation have the same dimensions. Example Show that the expression v = vo + at is dimensionally correct, where v and vo are the velocities and a is the acceleration, and t is the time Solution The right hand side [v] = L/T The left hand side L/T + L/T Therefore, the expression is dimensionally correct. Example Suppose that the acceleration of a particle moving in circle of radius r with uniform velocity v is proportional to the rn and vm. Use the dimensional analysis to determine the power n and m. Solution Let us assume a is represented in this expression a = k rn vm Where k is the proportionality constant of dimensionless unit. The right hand side n+m=1 and m=2 Therefore. n =-1 and the acceleration a is a = k r-1 v2 k=1 a= v2/r Exercise Part A: See if you can determine the dimensions of the following quantities: volume acceleration (velocity/time) density (mass/volume) force (mass × acceleration) charge (current × time) Check your answers You are correct if you wrote down: 1.Volume L3 2.acceleration (velocity/time) L/T2 3.density (mass/volume) M/L3 4.force (mass × acceleration) M·L/T2 5.charge (current × time) I·T Part B: Now find the dimensions of these: 1.pressure (force/area) 2.(volume)2 3.electric field (force/charge) 4.work (in 1-D, force × distance) 5.energy (e.g., gravitational potential energy = mgh 6.square root of area Check your answers pressure (force/area) 1. M·L-1·T-2 2. (volume)2 L6 3. electric field (force/charge) M·L·I-1·T-3 4. work (in 1-D, force × distance) M·L2/T2 5. energy (e.g., gravitational potential energy = mgh = mass × gravitational acceleration M·L2/T2 × height) 6. square root of area L Which one of the following quantities are dimensionless? 1-68 2-sin (68 ) 3-e 4-force 5-6 6-frequency 7-log (0.0034) What are the dimensions of the following? 1.[sin (wt)] 2.[3] 3.[force] 4.[height] 5.[frequency] 6.[displacement] 7.[area × volume] 8.[0.5 × volume]