Functional Groups 2
advertisement

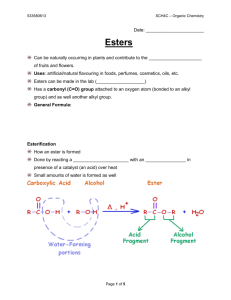
Functional Groups: - Aldehydes - Ketones - Organic Acids - Esters Aldehydes Group is always on a terminal C – no need to specify location by number Condensed it is symbolized by a -CHO group at the end of the formula C = O is known as a carbonyl group – Drives the physical and chemical properties of these compounds Aldehydes have characteristic scents and tastes – Cinnamon is an example Aldehydes Drop the –e from the end of the alkane and add –al; C=O is C #1 Name these two compounds: Methanal (formaldehyde) Butanal Ketones Like the aldehydes the Functional group is the carbonyl group C=O Contains an alkyl group on either side of the C=O – In aldehydes one side is an alkyl group the other is H Often used as a solvent – Acetone is one example Naming Ketones Number the C chain so C=O has the lowest # Drop the final e from the alkane name and add -one Name these compounds: 4-methyl-2-pentanone C 3-hexanone C-C-C-C-C o Properties Both aldehydes & ketones have A carbonyl functional group. C=O is polar & their compounds Are polar. This leads to: -Higher B.P’s than non-polar molecules -Lower than alcohols that can H-bond w/ each other -Can not H-bond between each other but can H-bond to solvents like water - Lower MW compounds are appreciably soluble in water Propanone Propanal Properties Butane Diethyl ether Propanal Propanone 1-propanol M.W. 58 58 58 58 60 B.P (deg.C) 0 35 49 56 97 Solubility g/100g H2O Insol. 8 16 Fully miscible Fully miscible Organic Acids Condensed form is written –COOH – A carbonyl group and a hydroxyl group bonded to C – Known as a carboxyl group A terminal functional group The carboxyl group is an organic acid. – It is the H on the end of COOH that is donated as a proton (H+) – What is left is the anion R-COO- Carboxylic acids ionize in water to form a proton and A carboxylate anion Naming Organic Acids The carboxyl group is a terminal group – no need to give it a number. It’s always on C #1 Drop the –e from the alkane name and add –oic acid Name this compound: Butanoic acid Naming Organic Acids Name this compound: Butanoic acid Name this compound: C-C-C-C-C-COOH C C 2,4-dimethylhexanoic acid Some Common Carboxylic acids HC=O OH CH3C=O OH Methanoic acid “formic acid” Ethanoic Acid “Acetic Acid” The sting of ants Vinegar C-C-C-COOH Butanoic acid Rancid butter smell Physical properties - Sharp or noxious smells - polar and can form 2 hydrogen bonds with themselves and with water C-C=O OH HO O=C- C C-C=O OH O H H This leads to high B.P. and high solubility in water - 1st four acids are totally miscible in water Comparison of properties Butane Diethyl ether Propanal Propanone 1-propanol ethanoic M.W. 58 58 58 58 60 60 B.P (deg.C) 0 35 49 56 97 118 Insol. 8 16 Fully miscible Fully miscible Fully miscible Solubility g/100g H 2O Esters Esters are derivatives of Carboxylic acids – Combination of an Alcohol and an Acid – The alcohol provides the alkyl portion of the ester name – Esters are polar but can not form hydrogen bonds with one another – BP’s of esters are lower than alcohols or acids with similar mass Physical properties Butanal Butanone Methyl 1-butanol ethanoate Propanoic Acid M.W. 72 72 74 74 74 B.P (deg.C) 76 80 57.5 118 141 Properties Esters have distinctive odors (flowers, flavors etc.) - Function of which alcohol is combined with which acid Alcohol Acid Ester Fragrance Ethanol Butanoic acid Ethyl butanoate Pineapple Pentanol Ethanoic acid Pentyl ethanoate Banana Octanol Ethanoic acid Octyl ethanoate Orange Methanol Salicylic acid Methyl salicylate wintergreen Methanol Butanoic acid Methyl butanoate apple Naming Esters Esters are not a terminal group but they are unique because of the carboxyl group therefore the carbonyl carbon is considered to be C #1 Name the group attached to the hydroxyl oxygen first (the alkyl group) Next name the carboxylate carbon chain, dropping the –e and adding –oate Written condensed format is RCOOR’ Name this compound: Ethyl butanoate Name this compound: O CH3-C-O-CH2-CH2-CH-CH3 CH3 3-methylbutyl ethanoate (Not IUPAC) 2-methylbutyl ethanoate What alcohols and acids produce the above esters? (Not IUPAC)