Chapter 6 Cellular Respiration
advertisement

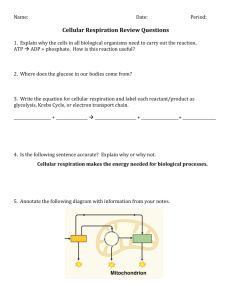
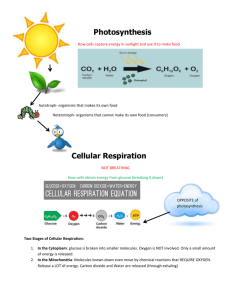
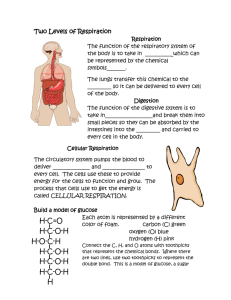
Chapter 6 - Cell Respiration Metabolism - the sum of all the chemical reactions that occur in the body. It is comprised of: anabolism – synthesis of molecules, requires input of energy catabolism – break down of molecules, releases energy.. aerobic – occurs in the presence of oxygen anaerobic – occurs in the absence of oxygen.. Energy Flow And Chemical Cycling In The Biosphere • Fuel molecules in food represent solar energy – Energy stored in food can be traced back to the sun • Animals depend on plants to convert solar energy to chemical energy – This chemical energy is in the form of sugars and other organic molecules.. • Those organisms that convert sun energy into food energy are producers. – autotrophs - the most common carry out photosynthesis • Those organisms that consume the autotrophs are consumers. – heterotrophs.. Chemical Cycling Between Photosynthesis And Cellular Respiration • The ingredients for photosynthesis are CO2 and H2O – CO2 is obtained from the air by a plant’s leaves – H2O is obtained from the damp soil by a plant’s roots • Chloroplasts rearrange the atoms of these ingredients to produce sugars (glucose) and other organic molecules – O2 is a by-product of photosynthesis • Both plants and animals perform cellular respiration – Cellular respiration is a chemical process that harvests energy from organic molecules and occurs in mitochondria • The waste products of cellular respiration, CO2 and H2O, are used in photosynthesis.. Sunlight energy Ecosystem Photosynthesis (in chloroplasts) Glucose Carbon dioxide Oxygen Water Cellular respiration (in mitochondria) for cellular work Heat energy We have been designed to liberate energy from food molecules by aerobic cellular respiration. This process is described as aerobic because oxygen is required. Why is oxygen required? During cellular respiration, hydrogen and its bonding electrons change partners from glucose to water. Oxygen is the final e- acceptor. Glucose Oxygen Carbon dioxide Water Energy • Chemical reactions that transfer electrons from one substance to another are called oxidationreduction reactions. – the loss of electrons (and hydrogens) is called oxidation – the gain of electrons (and hydrogens) is called reduction Oxidation [Glucose loses electrons (and hydrogens)] Glucose Oxygen Carbon dioxide [Oxygen gains electrons (and hydrogens)] Reduction Water When NAD (nicotinamide adenine dinucleotide) is reduced, a pair of hydrogen atoms donates a pair of e-, one of which then binds one proton and the other proton follows along = NADH + H+. We simplify this with NADH2.. Aerobic cellular respiration occurs in four stages: glycolysis transition reaction Krebs cycle electron transport pathway.. • Glycolysis – glucose must be “activated” by the addition of two phosphate groups P . The addition of the P also traps glucose within the cell. This process occurs in the cytosol. 2ADP + Pi 2ATP C6H12O6 glucose 2 C3H4O3 pyruvic acid 2NAD + 4H 2NADH2 2 Pyruvic acid Glucose • In animals if oxygen is not present to take the efrom NADH2, then the e- will be donated to pyruvic acid = Lactic acid pathway (anaerobic respiration). • The final product is lactic acid. This metabolic pathway only yields 2 ATP/molecule. 2 ADP+ 2 Glycolysis 2 NAD 2 NAD Glucose 2 Pyruvic acid + 2 H 2 Lactic acid • Various types of microorganisms perform fermentation – Yeast cells carry out a slightly different type of fermentation pathway = alcoholic fermentation – This pathway produces CO2 and ethyl alcohol 2 ADP+ 2 2 ATP 2 CO2 released Glycolysis 2 NAD 2 NAD Glucose 2 Pyruvic acid + 2 H 2 Ethyl alcohol • Transition reaction = pyruvic acid moves into the matrix of the mitochondrion. CO2 is cleaved off and at the same time Coenzyme A is added.Coenzyme A is derived from the vitamin pantotenic acid. NAD + 2H 2C3H4O3 + 2CoA pyruvic acid coenzyme A NADH2 2C2H3O-CoA + 2CO2 acetyl-CoA carbon dioxide CoA Acetic acid Pyruvic acid CO2 Acetyl-CoA (acetyl-coenzyme A) Coenzyme A Krebs Cycle • Acetic acid (2C) is added to oxaloacetic acid (4C) to form citric acid (6C). CO2 is enzymatically released. This occurs in the matrix of the mitochondria. 3NAD+6H 3NADH2 2C2H3O-CoA FAD+2H FADH2 2ADP+P 2ATP 4CO2 Input Output Acetic acid 2 CO2 ADP Krebs Cycle 3 NAD FAD Electron Transport System • e- are passed along a chain of molecules to O2, which acts as the final e- acceptor. – The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane – These ions store potential energy – When the hydrogen ions flow back through the membrane, they release energy 34 ADP+Pi 2 H+ + 2e- + ½ O2 34 ATP H2O • If the last member of the chain remained in a reduced state, it would be unable to accept more e-. Etransport would then progress only to the next-to-last molecule. This process would continue until all of the elements of the chain remained in the reduced state. At this point, the system would stop and no ATP could be produced in the mitochondrion. With the system incapacitated, NADH2 and FADH2 could not become oxidized by donating their electrons to the chain and, through inhibition of Krebs cycle enzymes, no more NADH2 and FADH2 could be produced in the mitochondrion. The Krebs cycle would stop and respiration would become anaerobic.. • Lipids and proteins can also be used in aerobic Food respiration. Polysaccharides Sugars Glycerol Fats Fatty acids Proteins Amino acids Amino groups Glycolysis AcetylCoA Krebs Cycle Electron Transport Lipogenesis • Excess glucose does not complete respiration but instead is converted into glycerol and fatty acids. The acetyl-CoA subunits from the transition reaction are added together to produce fatty acids. This occurs primarily in adipose tissue and the liver.. Lipolysis • Triglycerides are hydrolyzed into glycerol and free fatty acids (FFA) by lipolysis. • In some tissues glycerol can be converted into phosphoglyceraldehyde. • FFAs are a major energy source and are metabolized by b-oxidation.. Amino Acids • Excess amino acids (a.a.) in the diet are not simply stored as additional protein – instead they are deaminated and the carbon skeleton is either respired or converted to carbohydrates or fats. • Adequate amounts of amino acids are required for growth and repair. Some a.a. can be make by rearranging parts of carbohydrates and essential a.a. A new amino acid can be obtained by transamination. – Amine group (NH2) transferred from one amino acid to form another amino acid and a keto acid. – Catalyzed by a specific enzyme (transaminase).. • Excess amino acids are processed for excretion by oxidative deamination. The amine group is removed and converted to urea, which is then excreted by the kidneys. • Not all cells can use glucose as the energy source. • Blood contains a variety of energy sources: – Glucose and ketone bodies, fatty acids, lactic acid, and amino acids. • Different tissues preferentially use different energy molecules. – Blood [glucose] maintained as many organs spare glucose. • Why??