3 mol H 2 - Cloudfront.net
advertisement

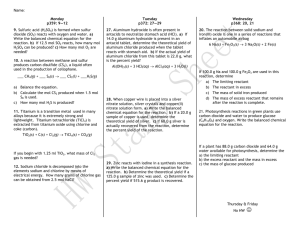
Example of a mole to gram (2-step) stoichiometry problem The Reaction: 6 CO2 + 6 H2O 1 C6H12O6 + 6 O2 • Question: If you have 5 moles of H2O how many grams of oxygen can you produce? • Conversion: 5 moles H2O to grams of O2 Start with the amount given in the question. Convert from moles to grams. Multiply by molar mass of O2 (16x2 = 32 g O2/mol) 5 mol H2O X 6 mol O2 = 5 mol O2 X 32 g O2 = 6 mol H2O 1 mol O2 Multiply by the mole ratio from the balanced equation with the unknown in the numerator. This cancels the given moles. 160 g O2 This is the answer. Notes: What is percent yield? • Percent yield describes the efficiency of a chemical reaction. • It is a comparison between the actual yield and the theoretical yield. • Calculate the percent yield: actual yield theoretical yield X 100% = percent yield •• Calculate percent yield: Calculatethe the percent yield: actual = percent yield yield actualyield yield X 100% X 100% = percent theoretical theoreticalyield yield You try 1: The Thetheoretical theoreticalyield yield 200when g. The yield 150 g.and Example: of is silver youactual combine 8g iscopper What is the ispercent yield? 1.7g AgNO 1.08 g Ag. The actual yield is 0.92 g Ag. What is the 3 percent yield? You try 2: The theoretical yield is 50 g. The actual yield is 25 g. WhatAgis theXpercent 0.92g 100%yield? = 85% 1.08 g Ag You try 3: When 34 g ammonia are combined with excess oxygen, the theoretical yield of nitrogen gas is 28 g. The actual yield is 22 g. What is the percent yield of nitrogen gas? Notes: Percent Yield (to practice – p. 319 #6, p. 331 #34) • Example: hydrogen and oxygen form water 2 H2 (g) + O2 (g) → 2 H2O (l) • Identify the limiting reactant and the theoretical yield of water (grams) when 10 g hydrogen react with 32 g oxygen. The actual yield is 27g water. • What is the percent yield? 1: Split into two stoichiometry problems. a)Step How manythe g Hquestion O form from 10 g H ? 2 2 Step 2: Solve both problems. Step 3: Compare answers. The smaller product amount is the theoretical yield. The limiting reactant is the reactant that b) How many g less H2Oproduct. form from 32 g O2? that formed Step 4: Percent yield – divide actual yield/theoretical x 100% Notes: Percent Yield (to practice – p. 319 #6, p. 331 #34) • Example: reaction of ammonia and oxygen 4 NH3 + 3 O2 → 6 H2O + 2 N2 • Identify the limiting reactant and the theoretical yield of Nitrogen (grams of N2) when 34 g ammonia (NH3 ) react with 64 g oxygen (O2). • The actual yield is 24 g N2. What is the percent yield? Step 1: Split the question intofrom two34stoichiometry problems. a) How many grams of N2 form g NH3 ? Step 2: Solve both problems. Step 3: Compare answers. The smaller product amount is the theoretical yield. The limiting reactant is the reactant that b)that How many grams of N2 form from 64 g O2? formed less product. Step 4: Percent yield – divide actual yield/theoretical x 100% N2 + 3 H2 2 NH3 1. What is the mole ratio of H2 to NH3? 3 mol H2 2 mol NH3 2. How many moles of H2 are needed to produce 10 moles NH3? 10 mol NH3 X 3 mol H2 = 2 mol NH3 15 mol H2 3. How many moles H2 are needed to produce 170 grams NH3? (molar mass of NH3 is 17g/mol) 170g NH3 ÷ 17 g/mol = 10 mol NH3 X 3 mol H2 2 mol NH3 = 15 mol H2 4. How many grams H2 are needed to produce 170 grams NH3? (NH3 =17g/mol; H2 = 2g/mol) 170g NH3 ÷ 17 g/mol = 10 mol NH3 X 3 mol H2 =15 mol H2X 2 g/mol H2 = 30 g H2 2 mol NH3 Notes: Limiting Reactant & Product Yield • Limiting reactant = the substance that controls the quantity of product that can form in a chemical reaction. – The limiting reactant runs out first. • Excess reactant = the substance that is not used up completely in a reaction. • Product Yield = the amount of product formed • Theoretical Yield = amount of product predicted from calculations. Think, Pair, Share: • What was the limiting reactant in your group’s s’more experiment? • What were the excess reactants? Notes: Limiting Reactant & Product Yield • Example 3: Copper and Silver Nitrate Cu (s) + 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq) • Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 128 g Cu reacts with 170 g AgNO3 . • Step 1: Separate the question into two stoichiometry problems: • How many grams Ag will be produced from 128 g Cu? • How many grams Ag will be produced from 170 g AgNO3? • Step 2: Solve both problems. • Step 3: Compare your answers. The smaller answer has the limiting reactant and is the theoretical yield. Notes: Limiting Reactant & Product Yield • Example 3: Copper and Silver Nitrate Cu (s) + 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq) • Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 128 g Cu reacts with 170 g AgNO3 . • How many grams Ag will be produced from 128 g Cu? • How many grams Ag will be produced from 170 g AgNO3? Practice: Limiting Reactant & Product Yield • Show all your work on your own sheet of paper. • Pre-Lab Problems: Copper and Silver Nitrate Cu (s) + 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq) 1. Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 64 g Cu reacts with 170 g AgNO3 . 2. Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 6.4 g Cu reacts with 8.5 g AgNO3 . 3. Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 32 g Cu reacts with 340 g AgNO3 . • • • Step 1: Separate the question into two stoichiometry problems: Step 2: Solve both problems. Step 3: Compare your answers. The smaller answer has the limiting reactant and is the theoretical yield. Check Your Understanding: 3e • Copper and Silver Nitrate Single Displacement Reaction: Cu (s) + 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq) 1. What is the mole ratio of silver (Ag) to copper (Cu) in the balanced equation? 2. What is the mole ratio of silver (Ag) to silver nitrate (AgNO3) in the balanced equation? 3. How many grams Ag will be produced from 640 g Cu? 4. How many grams Ag will be produced from 1700 g AgNO3? 5. Identify the limiting reactant and the theoretical (calculated) yield of silver (Ag) in grams, when 640 g Cu reacts with 1700 g AgNO3 .