Excited-State Processes
advertisement

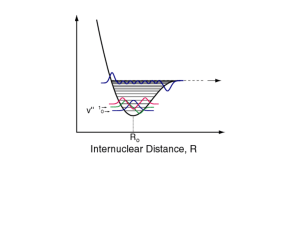
Reactive Intermediates Want to see time development of excited states and free radicals Excited states and free radicals act as individual chemical species during their existence. They are species of particular interest because of their high energy content. If you can capture their energy content, you can do chemistry that you cannot do in ground states. How to Utilize the Energy Content? If excited states channel their energy into specific bonds, then photochemistry can occur. If scavengers or quenchers can find the excited state or free radical in time, then the electronic or chemical energy can be captured by the, ordinarily, stable scavenger or quencher. Different Actions of Scavengers Direct capture of free radicals. Repair of damage caused by radicals. This second mechanism is important for the repair of damage by free radicals in biological systems. Motivations Oxidative stress • Alzheimer’s disease • Biological aging Basic issues • Neighboring-group effects • Details of oxidative scheme Radical Repair and Antioxidants R-S-H 1 H N-Acetyl-L-Methionine Amide STABILIZATION OF SULFUR RADICAL CATIONS VIA INTRAMOLECULAR SULFUR-NITROGEN AND SULFUR-OXYGEN BOND FORMATION Met-Gly Gly-Met Characterizing Excited States Excited states are not stationary states when consideration is made of the electromagnetic field. Therefore, excited-state processes are of primary significance. What happens to the energy when matter absorbs sunlight or UV? Gin and tonic glows under “black light” fluorescence Things heat up when sitting in the sun – radiationless transitions Some objects, like TV screens, glow after use or after the light is turned off – phosphorescence Objects appeared colored under visible light – differential absorption Excited-State Processes (Intramolecular) Fluorescence (fast radiative process) Phosphorescence (slow radiative process) Radiationless Transitions • Internal Conversion (transition with no spin flip) • Intersystem Crossing (transition with spin flip) • Vibrational Relaxation (heat produced) Photochemistry (bonds broken) Photoionization (no bonds broken, e- ejected) Photochemistry Jabłonski - diagram ISC S1 IC excited singlet state singlet ground state T1 fluorescence vibrational relaxation S1 ISC S0 (heat) phosphorescence S0 excited triplet state T1 State Picture Orbital Picture Radiationless Transitions Showing Nuclear Contributions “Stokes” shift Absorption vs Emission E = hc / Lifetimes & Quantum Yields Triplet states have much longer lifetimes than singlet states In solutions, singlets live on the order of nanoseconds or 10’s of nanoseconds Triplets in solution live on the order of 10’ or 100’s of microseconds Triplets rarely phosphoresce in solution (competitive kinetics) Competitive Kinetics Intramolecular decay channels isc T p S0 Intermolecular decay channels T + Q S0 + Q’ d [T ] kisc[T] k p [T] kq [Q][T] dt [T] [T]0 exp k isc k p k q [Q]t Intermolecular Excited-State Reactions Energy Transfer A* + B A + B* Electron Transfer D* + A D+ + A D + A* D+ + A Hydrogen Abstractions Note: Have to have excited states that live long enough to find quenching partner by diffusion Absorpcja przejściowa – diagram Jabłońskiego. Transient Absorption ISC S2 Tn IC TA IC TA ISC S1 T1 A IC F P C IS S0 Important Types of Organic Excited States ,* states, particularly in aromatics and polyenes n,* states, particular in carbonyls S2 1,* S1 1n,* S0 isc T2 3,* T1 3n,* Example: Lowest electronic states of Benzophenone Weak Spin Interactions Triplet states, spin interactions are weak so excited triplet states can live for some time But they have lower energy than their corresponding singlet states with the same orbital configurations By Pauli Exclusion principle - no two electrons in the same system can have the same quantum numbers By Pauli Exclusion principle like spins avoid each other – correlation hole around each electron Photochemistry of Triplet States 3n,* states are particularly good in Habstractions: they act like free radicals Molecules in excited states are generally more reactive in electron-transfer reactions than are their ground states Excited-State Electron Transfer Because of the “hole” in the HOMO of the ground state, the excited states have a low-lying orbital available for accepting electrons D e LUMO HOMO Because of the electron in the highly excited orbital, e.g the LUMO of the ground state, the excited state is a good donor for elect. transfer e LUMO A HOMO Why Triplet Quantum Yield is high in Benzophenone? S2 1,* S1 1n,* isc T2 3,* T1 3n,* Lowest electronic states of Benzophenone S0 (1)1n,* states have small krad because of small orbital overlap (2) kisc is large because of low-lying 3,* and El-Sayed’s Rule Selection Rules for ISC El-Sayed’s Rule Intersystem crossing between states of like orbital character is slower than ISC between states of different orbital character. Energy Gap Law The rate of radiationless transitions goes as the exponential of the energy gap between the 0-0 vibronic levels of the two electronically excited states Rational of Energy Gap Law Related to the probability of undergoing muliphononic events which gets more difficult as the number of phonons increases Franck-Condon factors get smaller as the difference in nuclear excitations between electronically excited states increase Classical Franck-Condon Factor Demonstrated for absorption Quantum Mechanical Franck-Condon Factors Demonstrated for absorption Deuterium Effect Radiationless transitions, e.g. intersystem crossing, slow down with deuterium substitution Franck-Condon Factors again are involved Relevance to Kasha’s Rule Tend to go to lowest levels in each multiplicity Relatively small gaps between higher singlet states, so IC is fast Within a particular electronic manifold, the vibrational relaxation is fast – here you can go one phonon at a time and still almost conserve energy within the molecule Robinson-Frosch Formula Radiationless Transitions dP(nm)/dt = (42/h) |Vmn|2 FC (Em) Probability per unit time of making a radiationless transition from the initial electronic state n, to a set of vibronic levels in the electronic state m is equal to the product of three factors (1) The square of the electronic coupling element (2) The Franck-Condon factor between the vibrational levels of the initial electronic state and the final one (3) The density of final vibronic states Implications of Robinson-Frosch dP(nm)/dt = (42/h) |Vmn|2 FC (Em) Electronic matrix elements are usually a factor of a thousand less for transitions that change spin Franck-Condon factors favor small changes in vibrational quantum numbers Higher density of states favors faster transitions