THE FATE OF EXCITED ELECTRONIC STATES
advertisement
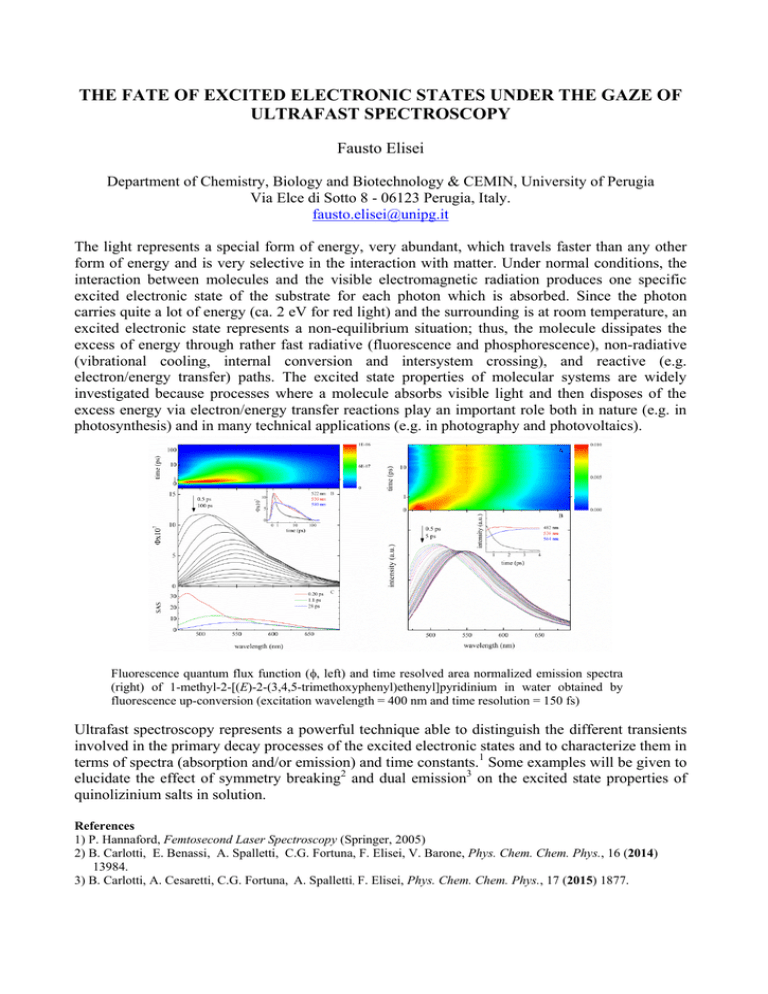
THE FATE OF EXCITED ELECTRONIC STATES UNDER THE GAZE OF ULTRAFAST SPECTROSCOPY Fausto Elisei Department of Chemistry, Biology and Biotechnology & CEMIN, University of Perugia Via Elce di Sotto 8 - 06123 Perugia, Italy. fausto.elisei@unipg.it The light represents a special form of energy, very abundant, which travels faster than any other form of energy and is very selective in the interaction with matter. Under normal conditions, the interaction between molecules and the visible electromagnetic radiation produces one specific excited electronic state of the substrate for each photon which is absorbed. Since the photon carries quite a lot of energy (ca. 2 eV for red light) and the surrounding is at room temperature, an excited electronic state represents a non-equilibrium situation; thus, the molecule dissipates the excess of energy through rather fast radiative (fluorescence and phosphorescence), non-radiative (vibrational cooling, internal conversion and intersystem crossing), and reactive (e.g. electron/energy transfer) paths. The excited state properties of molecular systems are widely investigated because processes where a molecule absorbs visible light and then disposes of the excess energy via electron/energy transfer reactions play an important role both in nature (e.g. in photosynthesis) and in many technical applications (e.g. in photography and photovoltaics). Fluorescence quantum flux function (, left) and time resolved area normalized emission spectra (right) of 1-methyl-2-[(E)-2-(3,4,5-trimethoxyphenyl)ethenyl]pyridinium in water obtained by fluorescence up-conversion (excitation wavelength = 400 nm and time resolution = 150 fs) Ultrafast spectroscopy represents a powerful technique able to distinguish the different transients involved in the primary decay processes of the excited electronic states and to characterize them in terms of spectra (absorption and/or emission) and time constants.1 Some examples will be given to elucidate the effect of symmetry breaking2 and dual emission3 on the excited state properties of quinolizinium salts in solution. References 1) P. Hannaford, Femtosecond Laser Spectroscopy (Springer, 2005) 2) B. Carlotti, E. Benassi, A. Spalletti, C.G. Fortuna, F. Elisei, V. Barone, Phys. Chem. Chem. Phys., 16 (2014) 13984. 3) B. Carlotti, A. Cesaretti, C.G. Fortuna, A. Spalletti, F. Elisei, Phys. Chem. Chem. Phys., 17 (2015) 1877.











