AP Biology Exam Review
advertisement
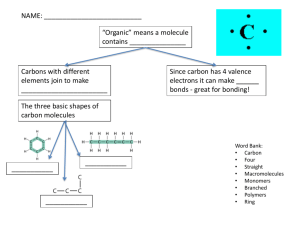
AP Biology Exam Review 60% multiple choice 40% free response Organizing life Atoms Molecules Organelles Cells Tissues Organs Systems Organism species Population Community Ecosystem Experimental design free response Problem Hypothesis Materials/procedure Control setup/baseline Independent and dependent variables Constants & variables Qualitative & quantitative data Data interpretation Conclusion Hypothesis Predictive May propose a method for testing the problem. Give a justification for the method of testing. Properties of life Metabolism: chemical pathways that are regulated Cellular organization Homeostasis: maintaining stable internal environment through controlled chemical reactions or metabolism for life functions (pH, temperature, etc) Properties of life Reproduction: capacity to develop from juvenile to adult stage with potential to replicate own DNA Asexual Sexual Properties of life Response to stimulus: able to react to external or internal changes Many responses to stimulus are result of enzymatic proteins. Chemical evolution of life production of small, reduced, carboncontaining compounds like formaldehyde and hydrogen cyanide. simple compounds reacted in the ocean to form the mid-sized molecules called sugars, amino acids, and nitrogenous bases Chemical evolution of life building block molecules linked together to form molecules found in cells (proteins and nucleic acids) single molecule acquired the ability to make a copies of itself Chemical evolution began to give way to biological evolution. Covalent Sharing of electrons Stable Forms hydrocarbons Polar covalent bonds Water molecule Leads to different water properties Ionic bonds Hydrogen bonds Weak individually Strength in multiple Hbonds Found between nucleotides Chemical reactions Properties of water Slightly positive and negative “poles” of water molecule form hydrogen bonds Frozen water molecules less dense, ice floats Water as ideal solvent Water as ideal solvent Water soluble protein Attracts water molecules pH: water dissociation pH scale Homeostatic control of pH (maintaining optimal pH levels) is necessary to sustain life. Ex: pH drop in blood = too much CO2 Organic chemistry Alkanes: hydrocarbons with only single bonds between C and H Alkenes: hydrocarbons with double bonds between C and H Alkynes: hydrocarbons with triple bonds between C and H Valence numbers Indicates the number of bonds that can be formed. Carbon structural molecules Isomers Molecules with the same molecular formula but different 3D configuration Functional groups Alcohol* Aldehyde Amine* Carboxylic acid* Ester Ether Ketone Methyl Phosphate* Polymers Most organic polymers form through dehydration synthesis. Most break apart by hydrolysis. Monosaccharides Single building block of sugars (carbohydrate) a-glucose, bglucose, fructose Disaccharides Polysaccharides Polysaccharides Starch: plant and algae storage, product of photosynthesis (a-glucose) Cellulose: structural polymer, product of photosynthesis (b-glucose) Chitin carbohydrate with an additional amine functional group that makes this molecule tough and water resistant exoskeletons of many insects fungal cell wall Lipids Ester linkage Why is this a saturated fat? Lipids Energy storage Insoluble in water C and H Saturated vs. Unsaturated Lipids: What is this structure? Lipids What are these structures? What proof is there that one of these structures makes up membranes? Lipids: What is this structure? Proteins: amino acid monomers Proteins: amino acid monomers Proteins Primary conformation: peptide bonds between amino acids Forms peptide chains Proteins Primary structure or conformation Notice the amino and the carboxyl terminus (ends) Proteins Secondary structure: hydrogen bonds between peptide chains Proteins Tertiary structure: Rgroup interactions, depends upon properties of R group Proteins: Quaternary structure Protein denaturation What can denature proteins? How cells “fix” denatured proteins Nucleic acids Nucleic acids are built from monomers of nucleotides. Nucleotides are adenine, thymine, cytosine, guanine, uracil. Ex: DNA, RNA, ATP, and GTP Nucleic acid DNA structure Notice the different types of bonds involved in the making of DNA DNA model Each nucleotide is made from deoxyribose sugar, phosphate, and nitrogen base. DNA is double stranded. Cells – 10% of test Prokaryotic and eukaryotic cells Membranes Subcellular organizations Cell cycle and its regulation Cell size Viruses not cells Bacteria, mitochondria, chloroplast all about the same size (evidence for endosymbiotic theory) Cell fractionation Prokaryotic cell Surface to volume ratio Governs size Membrane Eukaryotic – animal cell Eukaryotic – plant cell Freeze fracture Showing the “mosaic” of fluid mosaic model Singer and Nicholson Danielli proposed alternative model (protein-membraneprotein) Membrane fluidity Membrane structure Diffusion: entropy Osmotic balance Guard cells, excretory system, transpiration, translocation Osmotic balance Sodium-potassium pump Transport Passive vs. active transport Passive: osmosis Active transport: establishing proton gradient of electron transport chain Proton pump: auxin transport, electron transport chain Cotransport Translocation (phloem source to sink) Cell cycle Mitosis lab 500 cells = interphase = 50% 100 cells = prophase = 10% 150 cells = metaphase = 15% 150 cells = anaphase = 15% 100 cells = telophase = 10% Mitosis Mitosis Binary fission Asexual reproduction in prokaryotic cells Other examples of asexual reproduction: budding, regeneration, vegetative propagation Cell cycle control Requires various checkpoints and Cdk (cyclindependent kinase) protein to detect levels of cyclin Density Density dependent cellular growth vs. density independent cancerous growth