Cell Wall Synthesis Inhibitors
advertisement

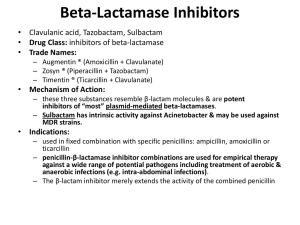
Cell Wall Synthesis Inhibitors By S.Bohlooli, PhD Inhibitors of Cell Wall Synthesis Penicillins Cephalosporins Monobactams Carbapenems Glycopeptides Other Cell Wall- or Membrane-Active Agents Chemical Structure Chemical Structure Penicillins In 1928, Alexander Fleming was researching vaccines and noticed a culture of staphlococci had undergone lysis from contamination with a mold Fleming finally isolated the mold, Penicillium notatum, and found that the fluid beneath it possessed antibacterial properties Basic structure consists of thiazolidine ring connected to b-lactam ring, to which is attached a side chain Structure of penicillins and products of their enzymatic hydrolysis. Chemical structure ,major variants Chemical structure ,major variants Cell envelope of a gram-negative bacterium Biosynthesis of cell wall peptidoglycan Mechanism of Action Interferes with last step of bacterial cell wall synthesis, causing cell lysis Bactericidal Only effective against rapidly growing organisms that synthesize a peptidoglycan cell wall Inactive against mycobacteria, fungi, viruses Mechanism of Action way of being bactericide Penicillin binding proteins Inhibition of transpeptidase Enzymes involved in forming cross linkages between peptidoglycan chains inactivated by penicillin Hinders last step in formation of cross links needed for cell wall integrity Autolysins Normally degrade cell wall, but penicillin prevents new synthesis Antibacterial Spectrum Natural penicillins Antistaphylococcal penicillins Extended spectrum penicillins Antipseudomonal penicillins Natural Penicillins Penicillin G (Benzylpenicillin) Gram +/- cocci, Gram + bacilli, spirochetes, anaerobes Susceptible to inactivation by b-lactamase Penicillin V Similar spectrum More acid stable than Pen G Higher minimum inhibitory concentration Bacteria Susceptible to Penicillin Strep pneumonia Gonorrhea Major cause of bacterial pneumonia in all ages Neisseria gonorrhea and meningitidis Syphilis Treponema pallidum Antistaphylococcal penicillins Methicillin Nafcillin Oxacillin Dicloxacillin All are penicillinase-resistant penicillins used in penicillinase-producing staphylococci Methicillin-resistant staph aureus Extended Spectrum Penicillins Ampicillin Amoxicillin Activity against haemophilus influenza, proteus mirabilis, E. coli, and neisseria species Resistance due to plasmid mediated penicillinase May use clavulanic acid or sulbactam to extend the antibacterial activity Antipseudomonas Penicillins Carbenicillin Ticarcillin Piperacillin Azlocillin Mezlocillin Effective against pseudomonas aeroginosa and other gram negatives Penicillins and Aminoglycosides Synergistic Inactivate each other if placed in same IV fluid bag because of positively charged aminoglycosides and negative penicillins Specific Indications for Penicillin Narrow-spectrum bactericidal Most Gm+ cocci and rods and anaerobes Intravenous: Pen G (potassium or sodium) Intramuscular: Benzathine or procaine Pen G Oral: Pen V Pen V, G well distributed in soft tissues Bone level is fraction of plasma concentration Excreted primarily via kidneys Bacterial Resistance b-lactamase activity 1. • • Decreased permeability to drug Altered penicillin binding proteins 2. 3. • 4. Hydrolyzes cyclic amide bond of b-lactam ring Usually acquired by transfer of plasmids May require greater antibiotic concentrations Efflux b-lactamase Inhibitors Clavulanic acid: product of streptomyces clavuligerus Irreversibly binds b-lactamases and inactivates them Augmentin: amoxicillin + clavulanic acid Timentin: ticarcillin + clavulanic acid Unasyn: ampicillin + sulbactam Pharmacokinetics Administration Absorption Oral, IV, IM Amoxicillin most completely absorbed Pen G absorption impeded by food in stomach Administered at least 1–2 hours before or after a meal. Distribution All cross placenta Minimal penetration into bone and CSF Pharmacokinetics (con’t) Metabolism Minimal, except in renal failure Excretion Kidney Adjust dose in renal compromise Probenecid inhibits penicillin secretion Adverse Reactions Hypersensitivity- 1-10% patients treated Penicilloic acid- hapten for immune reaction Urticaria to angioedema to anaphylaxis b-lactam cross reactivity Diarrhea- disruption of flora- ampicillin Nephritis- methicillin Neurotoxicity- intrathecal or seizure disorders Platelet dysfunction- carbenicillin, ticarcillin Cation toxicity- watch sodium (congenstive heart failure) and potassium (cardiac toxicity esp in renal failure pts) Skin rashes-ampicillin and amoxicillin Allergic Reactions Acute (< 30 min) Accelerated (30 min-48 hrs) Urticaria, angioedema, bronchoconstriction, GI, shock Urticaria, pruritis, wheezing, mild laryngeal edema, local inflammatory reactions Delayed (> 2 days) Skin rash Oral glossitis, flurred tongue, black and brown tongue, cheilosis, severe stomatitis with loss buccal mucosa Allergic Reactions Mild: Diphenhydramine 25-50 mg IV/IM/PO Severe: Epinephrine 0.03-0.05 mg Skin tests: benzylpenicilloyl-polylysine Cephalosporins Cephalosporium acremonium, first source, isolated in 1948 b-lactam antibiotics Related to penicillins structurally and functionally More resistant to b-lactamases Antibacterial Spectrum First generation Second generation Third generation Fourth generation Increased generation number, increased gram negative bacterial susceptibility, increased blactamase resistance, decreased efficacy against gram + First Generation Gram + cocci, gram - bacilli, oral anaerobes Staphylococcus aureus, Proteus mirabilis, E. coli, Klebsiella pneumonia Cefazolin, Cephalothin (parenteral) Cephalexin, Cefadroxil, Cephradine (oral) Second Generation Less active against G+, more GHaemophilus influenzae, Enterobacter aerogenes, Neisseria Cefaclor, Cefuroxime axetil (Oral) Cefamandole, Cefonicid, Cefuroxime, Cefotetan, Ceforanide (Parenteral) Cefoxitin- Bacteroides fragilis Used with aminoglycosides for G - bacilli Third Generation More Gm – bacilli, Serratia marcescens Cefixime (Oral) Cefotaxime Ceftizoxime Ceftazidime Cefoperazone Ceftriaxone Cefpodoxime Fourth Generation Similar spectrum to third generation More resistance to b-lactamases Cefepime hydrochloride Cephalosporins Active Against Methicillin-Resistant Staphylococci Ceftaroline fosamil Ceftobiprole medocaril Binding to penicillin-binding protein 2a Resistance Similar to penicillins Limited cross resistance with penicillin, except with staph and strep pneumonia Pharmacokinetics Administration Distribution Distributed well through body fluids Only third generation well into CSF Excretion Renal elimination Cefoperazone and ceftriaxone excreted through bile and feces General Therapeutic Uses Acute respiratory infection Gonorrhea and H. influenza Ceftriaxone Enterobacter, Serratia, E.Coli, Providencia, Salmonella First and second generation Third generation Gram negative meningitis and septicemia Third generation Adverse Effects Allergy- 15% cross sensitivity with Pen allergy Disulfiram-like effect Cefamandole, cefoperazone when ingested with alcohol block second step in oxidation and aldehyde accumulation Bleeding 1-2% without Pen allergy Cefamandole, cefoperazone- anti-vitamin K effects Renal, hepatic dysfunction Carbapenems Synthetic b-lactam antibiotics Differ from penicillin in sulfur atom of thiazolidine ring Imipenem Combined with cilastin- broadest spectrum blactam antibiotic available against penicillinaseproducing Gram + and -, anaerobes, and pseudomonas Resists b-lactamase hydrolysis Meropenem, doripenem, ertapenem Greater activity against gram-negative aerobes Carbapenems Intravenous use Penetrates well into CNS Excreted by kidneys Toxic metabolite may cause nephrotoxicity Administered with cilastin to prevent cleavage and toxic metabolite formation Nausea, vomiting, diarrhea, seizures, eosinophilia, and neutropenia Monobactams Narrow spectrum- enterobacteriaceae, pseudomonas; no gram + or anaerobic activity Resistant to b-lactamase Aztreonam IV or IM Phlebitis, skin rash, abnormal liver function tests Little cross reactivity with penicillin Vancomycin It binds firmly to the D-Ala-D-Ala terminus and inhibits transglycosylase. Narrow-spectrum against methicillin-resistant staphylococci and pseudomembranous colitis caused by clostridium difficile Prophylaxis for subacute bacterial endocarditis in penicillin allergic patients for high risk surgery Oral route only for P. colitis IV for systemic infections Vancomycin Minimal resistant bacteria, but not vancomycin resistant enterococci (VRE) Renal elimination Fever, chills, phlebitis at infusion site, rash with chronic administration, ototoxicity (cochlear damage above 80 mg/ml), nephrotoxicity Slow IV administration- fast causes histamine release (“red man syndrome”), hypotension Newer glycopeptide antibiotics Teicoplanin Dalbavancin It can be given intramuscularly Derived from teicoplanin Effectrive on methicillin-resistant and vancomycinintermediate S aureus Telavancin Derived from vancomycin active versus gram-positive bacteria including strains with reduced susceptibility to vancomycin Other Cell Wall- or Membrane-Active Agents Daptomycin Fosfomycin Novel cyclic lipopeptide Similar to that of vancomycin Active against vancomycin-resistant strains of enterococci and S aureus Analog of phosphoenolpyruvate Active against both gram-positive and gram-negative Cycloserine Bacitracin Mechanism of action of daptomycin Bacitracin Bacitracin inhibits cell wall synthesis by interfering with dephosphorlyation in cycling of the lipid carrier Effective against Gram positive microorganisms Topical application due to nephrotoxicity Often used for traumatic abrasions