File
advertisement

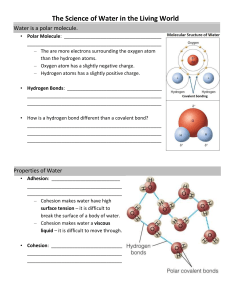
Chemical Properties of Water Water and Life Life on Earth began in water and evolved there for 3 billion years. Modern life still remains tied to water Cells are composed of 70%95% water •Water is found as a liquid over 71% of the earth •The abundance of water is a major reason Earth is habitable The structure of water Studied in isolation, the water molecule is deceptively simple Its two hydrogen atoms are joined to one oxygen atom by single covalent bonds H H O But the electrons of the covalent bonds are not shared equally between oxygen and hydrogen Not in notes*** This unequal sharing makes water a polar molecule Oxygen is more electronegative than hydrogen, so it has a greater pull on the electrons () () () () The polarity of water results in weak electrical attractions between neighboring water molecules These interactions are called hydrogen bonds () Hydrogen bond () () () () () (b) () () Polar Structure Electronegativity of H20 Water’s Life Supporting Properties The polarity of water molecules and the hydrogen bonding that results explain most of water’s life-supporting properties Water’s cohesive nature Water’s ability to moderate temperature Floating ice Versatility of water as a solvent The Cohesion of Water Water molecules stick together as a result of hydrogen bonding Microscopic tubes Cohesion is vital for water transport in plants Surface tension is the measure of how difficult it is to stretch or break the surface of a liquid Can support animals like “water striders” in ponds Surface Tension Water drops are round because all the molecules on the edge are pulled to the middle. Water will also be attracted to other polar substances This is called adhesion It is due to the polar nature of the water molecule Caused by adhesion, the water runs along the glass and does not fall straight. Capillary Action Glass has polar molecules. Glass can hydrogen bond. Attracts the water molecules. Some of the pull is up. Water moves up from ground using cohesion and adhesion. Transpiration pulls water out of ground into tree, water molecules cohere to each other and adhere to sides of xylem tube in tree. Describe an example of how cohesion, adhesion, & capillary action work in a living organism: Meniscus Water curves up along the side. This makes the meniscus. How water moderates temperature Because of hydrogen bonding, water has a strong resistance to temperature change Heat and temp Heat is the measure of the amount of kinetic energy in the environment and molecules and object has Temperature measures avg. energy of the molecules. How water moderates temperature Whenever 2 objects meet, the cooler object absorbs heat from the warmer object until they are the same temperature Water absorbs heat during warm periods, then the shore absorbs that stored heat during cooler periods. Despite the fact that water is losing/gaining heat energy, it is not necessarily changing temperature because it has a high specific heat. How water moderates temperature Water has a high specific heat Specific heat = the amount of heat that must be absorbed or lost to change the temperature of 1g of the substance 1°C How water moderates temperature Since water has a high specific heat, it will not change temperature much when it absorbs or loses heat This is because much of the absorbed heat is used to break hydrogen bonds, not increase the kinetic energy of the molecules How water moderates temperature So water can absorb and store large amounts of heat while only changing a few degrees in temperature Large bodies of water help to moderate temperature Earth’s giant water supply causes temperatures to stay within limits that permit life Evaporative cooling removes heat from the Earth and from organisms Compare the temperature changes (per season or per day) of coastal habitats to inland habitats: How water moderates temperature Water also has: High heat of fusion • The temp at which liquid turns solid High heat of vaporization • The temp at which liquid turns to gas The Biological Significance of Ice Floating When water molecules get cold, they move apart, forming ice A chunk of ice has fewer molecules than an equal volume of liquid water Liquid water Ice The density of ice is lower than liquid water This is why ice floats Hydrogen bond Ice Stable hydrogen bonds Liquid water Hydrogen bonds constantly break and re-form Figure 2.15 Change of State Dipole Structure Ice floats in water because all ice molecules are held in hexagons The center of the hexagon is open space, making ice 8% less dense than water. Since ice floats, ponds, lakes, and even the oceans do not freeze solid Marine life could not survive if bodies of water froze solid Floating ice insulates water below, preventing freezing: critical for ocean animals Maximum density: 3.98oC Below this temp, form hexagonal polymers and decrease density Above this, molecules are energetic, water behaves like other liquids - expanding when warm and contracting when cool Water as the Solvent of Life A solution is a liquid consisting of two or more substances evenly mixed The dissolving agent is called the solvent The dissolved substance is called the solute Ion in solution Salt crystal When water is the solvent, the result is called an aqueous solution Water is a good solvent because it is polar Ionic (salts) and polar (sugars) compounds dissolve readily in water Solvent Properties Water dissolves salts by surrounding the atoms in the salt molecule and neutralizing the ionic bond holding the molecule together Free water is water not attached to solute. Any time you add a solute you have less free water. What is the “rule” for solubility?