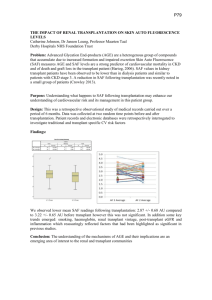
Neoplasm (melanoma) risk in transplant patients
advertisement
The role of immunosuppression and sunlight exposure in cancer formation with post-renal transplant patients By: Amanda Stevens Advisor: Dr. Boissonneault Overview • Why is this important to me? • Why should this be important to you? • Many people in our communities are immune compromised. • Immune suppression does not discriminate. Why and How to Immunosuppress? • One way of becoming immune compromised is by receiving a transplant and then being treated with immunosuppressant drugs to prevent allograft rejection. • These drugs can give you one of three outcomes: – Therapeutic effect – Undesired consequences of immunodeficiency – Nonimmune toxicity to other tissues Undesired consequences of immunodeficiency • All of the immunosuppressant drugs interfere with body’s ability to survey and destroy tumor cells. • This leads to an unchecked balance in the body which can produce tumors and cancers. • One specific cancer that kidney transplant recipients encounter frequently are skin cancers. 27% of deaths occurring at least 4 years post transplantation were caused by skin cancer! At 20 years post transplantation, cancer incidence is estimated to be 70% for renal transplant recipients! This is your natural immune system Types of immunosuppressant drugs given to RTRs • Grouped according to their MOA • Cyclosporine and Tacrolimus – Calcineurin inhibitors – A little over 20 years old • Mycophenolate Mofetil [MMF] (CellCept®) – Since 1995 – Reversible inhibitor of the enzyme inosine monophosphate dehydrogenase (IMPDH) Types of immunosuppressant drugs given to RTRs • Sirolimus and Everolimus – “TOR” Inhibitors – Blocks proliferation • Azathioprine – Use started more than 30 years ago – Incorporates itself into DNA – Broad myelocyte suppressant Types of immunosuppressant drugs given to RTRs • Corticosteroids – Introduced to the transplant community in the 1960’s – Inhibits the expression cytokines such as: IL-1, IL-2, IL-3, IL-6, TNF-ά, and γ-interferon. • Monoclonal and Polyclonal Antibodies – OKT3 (Monoclonal) – IgG – Humanized anti-CD25 Monoclonal Ab (Basiliximab and Daclizumab) – Atgam (Polyclonal) replaced by Thymoglobulin preparations. • New drugs on the way!!!! • Studies have tried to prove…. This is your immune system on drugs…immunosuppressants that is To sum up the drugs… • Berg and Otley did a study and the paper it produced said: – First, agents used during transplantation themselves may be carcinogenic – Second, by having chronic immunesuppression this creates a state in which natural surveillance and eradication of precancerous transformations are hindered! Types of cancers found in RTR • Some do not occur at an elevated rate: breast, colon, lung, and prostate. • Some ARE elevated including: esophagus, skin, liver, cervix, bladder, thyroid, and renal cells. • Focusing now on skin! Types of cancers found in RTR General Population Renal Transplant Recipients Breast, colon, lung, and prostate Esophagus, skin, liver, cervix, bladder, thyroid, and renal cells Basal Cell Carcinoma BCC>SCC at 2-4:1 Squamous Cell Carcinoma SCC>BCC at 3:2 Melanoma, Kaposi’s sarcoma, Merkel cell carcinoma Locations of cancer • SCC and BCC are normally found on constantly sun exposed areas: –Temples, forhead, lips, auricles, neck, and upper extremities. Things can look benign…. A squamous cell carcinoma in an RTR. http://www.captaincutaneum.com/science/squamous/images/squamous_01.jpg …or really bad… http://www.lib.uiowa.edu/hardin/md/pictures22/ dermnet/21_basal_cell_carcinoma_cancer_imi quimod0822057.jpg (top right) http://www.imr.gov.my/org/CRC/slide57f.jpg (bottom right) http://www.aad.org/education/students/_i mg/ActinicKerNoMelCancer13.jpg (left) Risk factors for cancer • No single factor can be pinpointed. • Having multiple risk factors will increase the incidence of skin cancers. • Risk factors include: – Being an older pt – Duration of immunosuppression – Male – Earlier age of transplantation – Higher dose of immunosuppresion – Genetic predispositions – Having skin types I, II, or II on Fitzpatrick scale – Significant exposure to UV radiation – HPV infection – Lower CD4+ cell counts naturally Quick Stats • Only 54% of renal transplant patients even remember getting advice on staying out of the sun. • Of those 54%, only 30% knew why it was important. • 27% of deaths occurring at least 4 years post transplantation were caused by skin cancer! • The average post-transplant neoplasm appears on average at approximately 5 years. • Only 5.6% of RTRs used sunscreen on a consistent basis prior to transplantation. • That number only increased to 36.7% after transplantation. What we can do to help! • Education – – – – Avoid sun exposure Use sunscreens Cover up Avoid “peak hours” (10a-4p) • Team approach • Primary prevention – Screenings (tumor markers, Immunoknow Assay, sCD30) • Early intervention In conclusion! • Educate ourselves! • Educate our pts! Thanks for your attention! Thanks Dr. B! References • • • • • • • • • • • • • • Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002; 47: 1-20. Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES. Skin cancer following transplantation: The Israel Penn International Transplant Tumor Registry experience. Transplant Proc 2005; 37: 962-963. Chu AC, Edelson RL. Malignant Tumors of the Skin. Arnold, 1999. Cupples SA, Ohler L. (Eds). Solid Organ Transplantation. Springer Publishing Company, 2002. Danovitch GM. (Ed). Handbook of Kidney Transplantation, 4th Edition. Lippincott Williams & Wilkins, 2005. Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, Blanc D, Humbert P, Chalopin JM. CD4 lymphocytopenia as a risk factor for skin cncers in renal transplant recipients. Transplantation 1998; 65 (9): 1270-1272. Euvrard S, Kanitakis J. Skin cancers after liver transplantation: what to do? J Hepatol 2006; 44: 27-32. Ginns LC, Cosimi AB, Morris PJ. Immunosuppression in Transplantation. Blackwell Science, 1999. Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Immunology, 5th Edition. W.H. Freeman and Company, 2003. Griffin MD, Kumar R. Multiple potential clinical benefits for 1ά,25-dihydroxyvitamin D3 analogs in kidney transplant recipients. J Steroid Biochem Mol Biol 2005; 97: 213-218. Halloran PF. Immunosuppressive Drugs for Kidney Transplantation. NEJM 2004; 351: 2715-2729. Israeli M, Yussim A, Mor E, Sredni B, Klein T. Preceeding the rejection: In search for a comprehensive post-transplant immune monitoring platform. Transpl Immunol 2007; 18: 7-12. Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Fauchald P. Transplant Proc 1999; 31: 1120. Laing ME, Kay E, Conlon P, Murphy GM. Genetic factors associated with skin cancer in renal transplant patients. Photodermatol Photoimmunol Photomed 2007; 23: 62-67. References cont. • • • • • • • • • • • • • • • Lewis KG, Jellinek N, Robinson-Bostom L. Skin cancer after transplantation: A guide for the general surgeon. Surg Clin N Am 2006; 86: 1257-1276. Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000; 143: 513-519. Moloney FJ, Almarzouqi E, O’Kelly P, Conlon P, Murphy GM. Sunscreen use before and after transplantation and assessment of risk factors associated with skin cancer development in renal transplant recipients. Arch Dermatol 2005; 141: 978-982. Nairn R, Helbert M. Immunology for Medical Students, 2nd Edition. Mosby Elsevier, 2007. Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Safety 2000 (Aug); 23 (2): 101-113. Ringborg U, Brandberg Y, Breitbart EW, Greinert R. (Eds). Skin Cancer Prevention. Informa Healthcare USA, Inc., 2007. Rodrigo E, Arias M. A practical approach to immune monitoring in kidney transplantation. Minerva Urol Nefrol 2007 (Sept); 59 (3): 337-352. Sayegh MH, Remuzzi G. Current and Future Immunosuppressive Therapies Following Transplantation. Kluwer Academic Publishers, 2001. SchmiederRE. Rening inhibitors: optimal strategy for renal protection. Curr Hypertens Rep. 2007 (Nov); 9 (5):41521. Seukeran DC, Newstead CG, Cunliffe WJ. The compliance of renal transplant recipients with advice about sun protection measures. Br J Dermatol 1998; 138: 301-303. Sharon, J. Basic Immunology. Williams & Wilkins, 1998. U.S. Food and Drug Administration, Office of Oncology Drug Products: FDA Licenses Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Recombinanat Vaccine (Gardasil) for the Prevention of Cervical Cancer and Other Diseases in Females Caused by Human Papillomavirus. Date of page: July 20, 2006. Available at: http://www.fda.gov/cder/Offices/OODP/whatsnew/gardasil.htm, accessed on 12/1/2007. Vachharajani TJ and Atray NK. Aging veterans and the end-stage renal disease management dilemma in the millennium. Hemodial Int. 2007 Oct.; 11(4): 456-60. Virella G. (Ed). Medical Immunology, 6th Edition. Informa Healthcare USA, Inc., 2007. Wong RF, Zeng G, Johnston SF, Voo K, Ying H. T cell-mediated immune responses in melanoma: implications for immunotherapy. Crit Rev Oncol Hematol 2002; 43: 1-11.