Density: Definition, Calculation & Practice Problems
advertisement

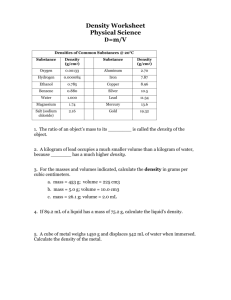
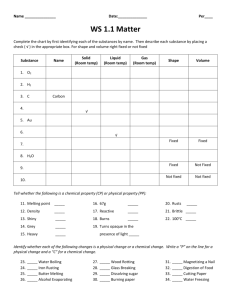
Density physical • Density is a _________ property of matter. • Density can be defined as the amount matter in a given ________. space of ________ • Example: Two objects have the exact same volume, but one is made of more matter. • Example: Which has more mass, a kilogram of feathers, or a kilogram of lead? • Density is a physical property of a substance that is used to identify that substance. Density of Lead Density of Lead Density of Lead • The size of the object does not change the density. The density of lead is always going to be the same. • Relative density: Matter that is less dense will float on matter that is more dense. • Calculating density: If you know the ________ and mass volume of an object, you can find ________ its density. The formula: density = mass/volume OR D= m -----v • Identifying your units! The base unit for mass (m) of an object is grams (g). The base unit for volume of a solid is cubic centimeters (cm3). The base unit for volume of a liquid is milliliters (ml) • How to find volume: Regular solid: a solid which the sides can be measured using a ruler to get the volume. V= L x W x H Irregular solid: a solid with sides that are not capable of being measured with a ruler. Example: odd shaped rock For this type of object we use WATER DISPLACEMENT Water Displacement To Find the volume of an irregular solid: 1. Using a graduated cylinder fill with water to a specific amount such as 100 ml. 2. Slowly lower object in to the graduated cylinder (the water will rise). 3. Subtract the final amount from the initial amount and that will be the volume of the object. 4. Convert from ml to cm3. Practice Problem 1 • A 800g rock has a volume of 8cm3. What is the density of the rock? Practice Problem 2 • A piece of wood that measures 3.0 cm by 6.0 cm by 4.0 cm has a mass of 80.0 grams. What is the density of the wood? Would the piece of wood float in water? (volume = L x W x H) Practice Problem 3 • Archimedes was commissioned to determine if the crown given to the king was pure gold or not. If the crown had a mass of 882 grams and displaced 50 mL of water, was the crown pure gold? • The density of Pure Gold is 19.3 g/cm3.