Density - TeacherWeb
advertisement

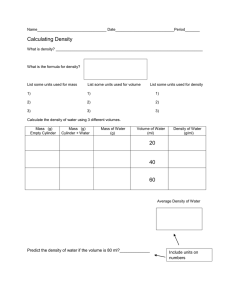
DENSITY Notebook Page 24 Definition • Density is the measure of how much mass is contained in a given volume. • (Or: how close a substance’s molecules are) • The density of a pure substance is always the same, no matter how much of it you have. • Ex: Gold is 19.3 g/cm3 at room temperature • The density of a substance can change when its temperature changes. • Why? Why is density important?? • Density is a physical property of matter. • Density has been used for centuries to identify substances. Calculating Density • Density = Mass / Volume or D = M • The metric unit is grams per cubic centimeter or grams per milliliter. • The symbol for density is the Greek letter Rho (ρ) • Use the magic triangle if you need help isolating for a variable • Ex: solve for V Calculating Density • Calculate the volume of “regularly shaped” objects by multiplying length x width x height. • Then divide the mass by the volume • Remember, it must be in g/cm3 lxwxh 6m x 6m x 8m = 228 m3 228 m3 = ______ cm3 D=m/V D = 600 g cm3 D= Calculating Density • If an object is not “regularly shaped”, then it can be placed in water. • The amount of water displaced is the volume of the object. Use grams per milliliter. • Use .01 kg for the mass of the rock: .01 kg = _______ g D=m/V D = ________g mL D= g / mL Sink or Float? • All substances are compared to water • The density of water is always 1.0 g/mL. • If a substance floats in water, than it is less dense than water. Ex: D = 0.6 g/mL • If a substance sinks in water, than it is more dense than water. Ex: D = 2.3 g/mL Duh! That means it “ Sinks like a stone!!!” Hey, let’s do a teacher demo!! • Which is denser, a can of Pepsi or a can of Diet Pepsi? • No, you can’t drink it!! • Yay! You are done with notes!!! Density Lab pg ____ • Obj: The student will learn to identify an unknown substance by calculating its density. • Materials: Density blocks (or cylinders), metric ruler, graduated cylinder with 50 mL water, digital scale, calculator. Procedure 1. 2. 3. 4. 5. 6. With a partner get a digital scale, a calculator, and either 3 density blocks or 3 density cylinders. Determine if you need a metric ruler or a graduated cylinder with 50mL of water. How do you know which? Calculate the density of each block or cylinder. Compare it to the list on the board and identify what each block/cylinder is made of. YOU WILL PROBABLY NOT BE ABLE TO MATCH THE NUMBERS ON THE LAST SLIDE. THAT’S OK, USE YOUR OBSERVATIONS TO HELP YOU PICK THE MOST LOGICAL. Those numbers were calculated by pros using high-tech equipment! Data Table - Blocks Block 1 Color Texture Length Width Height Volume (cm3) Mass Density Identity of substance Block 2 Block 3 Data Table - Cylinders Cylinder 1 Color Texture Volume (mL) Mass Density Identity of substance Cylinder 2 Cylinder 3 Calculate Density – Identify Substance Cylinders Density Blocks Density Copper 8.9 Copper 8.9 Brass 8.0 Brass 8.0 Pyrex glass 2.23 Steel 7.6 Rubber 1.15 Polypropylene 0.90 Acrylic 1.4 Acrylic 1.4 Aluminum 2.7 Aluminum 2.7 Nylon 1.14 Nylon 1.14 PVC (polyvinyl chloride) 1.50 PVC (polyvinyl chloride) 1.50 Teflon 2.19 Wood (pine) 0.50 Tecaform 1.41 Wood (lignum vitae) 1.33 Wood (poplar) 0.45 Wood ( poplar 0.45 Wood (Oak) 0.75 Wood (Oak) 0.75