Chemical Reactions - FHS gators love Science
advertisement

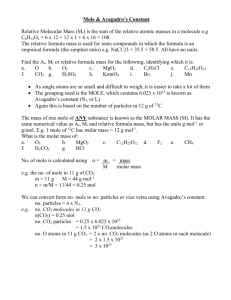
Chemical Reactions Chapter 7 What type of change is happening in the picture? When charcoal burns, it changes into other substances while producing heat and light. Burning is a chemical change Describing Reactions 7.1 One way to describe a change of state is to describe what is present before and after the change. In a chemical reaction, the substances that undergo change are called reactants. The new substances formed as a result of that change are called products. ◦ In the picture, the reactants are the carbon in the charcoal, and the oxygen in the air. ◦ The product is CO2 gas. Chemical Equations Reactants Products To describe burning of charcoal: Carbon + Oxygen Carbon Dioxide C + O2 CO2 A chemical equation is a representation of a chemical reaction in which the reactants and products are expressed as formulas Using Equations to Represent Reactions What happens to the products in a chemical reaction? During a chemical reaction, the mass of the products is always equal to the mass of the reactants. This is called the Law of Conservation of Mass, that mass is neither created nor destroyed. This law was established by Antoine Lavoisier Conservation of Mass Equation reads “one carbon atom reacts with one molecule of oxygen and forms one molecule of carbon dioxide”. If you have 6 C atoms, they will react with 6 O2 molecules to form 6 CO2 molecules The equation has the same number of atoms on each side of the equation Conservation of Mass Some chemical reactions are powerful enough to propel a space craft. ◦ Rocket fuels contain a compound called hydrazine, N2H4 ◦ When hydrazine burns in the presence of oxygen, the reaction produces nitrogen, water vapor, and heat. ◦ You can describe this reaction by writing a chemical equation: ◦ N2H4 + O2 N2 + H2O Balancing Equations N2H4 + O2 N2 + H2O If we count the atoms on both sides, we will see that the # of atoms on the left are not equal to the # of atoms on the ride side of the equation. This equation is NOT balanced In order to show that mass is conserved during a reaction, a chemical equation must be balanced. You balance a chemical equation by changing the coefficients (# in front of the formula) Balancing Equations No coefficient- is an understood “1” First, count the number of atoms of each element on each side of equation Starting with metals, change coefficients in front of formulas until balanced. Try ◦ Na + H2O NaOH + H2 HCl + CaCO3 CaCl2 + CO2 + H2O Steps to Balancing Equations Write a balanced equation for the reaction between copper and oxygen to produce copper (II) oxide, CuO. ◦ Steps: Write equation with reactants on the left and products on the right ◦ Cu + O2 CuO Balance atoms ◦ 2Cu + O2 2CuO MATH PRACTICE Balance the following chemical equations: ◦ H2O2 H2O + O2 ◦ Mg + HCl H2 + MgCl2 Math Practice How many shoes do you have? Shoes are counted in pairs. How many eggs are in a dozen? Bottle rockets in a gross? Pair, dozen, gross are all UNITs we use to count. How do chemists count particles? DEMO Counting Chemicals Chemists need to be able to count atoms or molecules. These units are too small to be counted, so chemists have another way to count them. Chemists use a unit called the mole (No, not that kind of mole!) A mole is an amount that contains 6.02 x 1023 particles of that substance ◦ Aka “Avogadro’s #’ ◦ Particles: atoms, molecules or ions ◦ Ex: 1 mole of Fe (Iron) contains 6.02 x 1023 atoms of iron. Counting Moles Does a dozen eggs weigh the same as a dozen oranges? Demo ◦ A mole of carbon has a different mass than a mole of sulfur The mass of one mole of substance is called a molar mass The molar mass for chemicals is the same as the atomic mass. ◦ Ex: Carbon’s mass is 12 amu or 12 grams per one mole of carbon 12 grams C or 1 mole C 1 mole C Molar Mass 12 grams C For a compound, add the atomic masses of its components atoms ◦ EX: CO2 ◦ 1 atom of Carbon x 12 g ◦ + 2 atoms of Oxygen x 16 g 44 grams of CO2 per one mole of CO2 “Molar mass” is the same as “formula mass” You can use this molar mass to convert moles of a substance to mass and vice versa. 44.0 g CO2 1 mol CO2 or 1 mol CO2 44.0 g CO2 Mole Mass Conversions Suppose you have 55 g of CO2. You can use the g/mol conversion factor to calculate how many moles: ◦ 55.0 g CO2 1 mol CO2 1.25 mol CO2 44g CO2 ◦ You can also convert from moles back to grams ◦ 2 mol CO2 44g CO2 1 mol CO2 88.0 g CO2 Mole-Mass Conversions Types of Reactions 7.2 How do you classify matter? ◦ (solid, liquid, gas – remember?) Chemical reactions are also classified into different types: ◦ ◦ ◦ ◦ ◦ Synthesis Decomposition Single-replacement Double-replacement Combustion Classifying Reactions Synthesis reactions are reactions in which 2 or more substances react to form a single substance ◦ A + B AB (like a MARRIAGE) ◦ Ex: 2 Na + Cl2 2 NaCl ◦ Video Decomposition reactions are reaction in which one substance is broken down into two or more simpler substances (opposite of synthesis) ◦ AB A + B (like a DIVORCE) ◦ Ex: 2H2O 2 H2 + O2 ◦ Video Synthesis & Decomposition Here is another example of a synthesis reaction Another view of a decomposition reaction Single Replacement is a reaction where 1 element takes the place of another element in a compound ◦ A + BC B + AC (like a LOVE TRIANGLE) ◦ Ex: Cu +2Ag(NO3) 2Ag + Cu(NO3)2 ◦ Video Double Replacement two different compounds exchange positive ions and form 2 new compounds ◦ AB + CD AD + CB (like DO-Si-DO and change partners) ◦ Ex: Pb(NO3) + 2KI PbI2 + 2 KNO3 ◦ Video ◦ With precipitation Single and Double Replacment Single Replacement Reactions Combustion reaction one in which a substance reacts rapidly with oxygen, producing heat and light ◦ Ex: CH4 + 2O2 CO2 + 2 H2O ◦ Always react with oxygen and usually produces CO2 , gas and water Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide) Many reactions can be classified by more than one type. Combustion Oxidation-reduction reaction: a reaction where electrons are transferred from one reactant to another, aka redox Ex: Calcium reacts with oxygen to produce calcium oxide Oxidation 2Ca + O2 2CaOCan occur without ◦ The product (CaO is composed of ions where the reactants were neutral atoms) oxygen ◦ When calcium reacts with oxygen, each neutral atom loses electrons to form Ca+2 ions Ca Ca2+ + 2e- When an element loses electrons during a chemical reaction it is called oxidation The calcium lost electrons so it was oxidized Oxidation-Reduction (Redox) Reduction is the process where an element gains electrons Each neutral oxygen atom gains two electrons becoming O2- ion O + 2e- O2 The oxygen gained electron and has been reduced OILoccur RIG together ◦ Oxidation and reduction always Oxidati ◦ If one element loses electrons another element on HAS to gain them Is Loss Reducti on Oxidation-Reduction (Redox) Is Gain • 1. 2. 3. 4. 5. State the type, classify and balance the following reactions: _Pb(NO3)2 + _HCl _PbCl2 + _HNO3 _C2H6 + _O2 _CO2 + _HOH _Ca + _HCl _CaCl2 + _H2 _Hg + O2 HgO _SO2 + _O2 _SO3 Do you see a Redox reaction? Mixed Practice Energy Changes in Reactions 7.3 Where does the heat come from when you light a propane grill? ◦ C3H8 + 5O2 3CO2 +4H2O + Heat ◦ This equation shows that the heat released in the reaction came from the reactants. ◦ Chemical energy is the energy stored in the chemical bonds of a substance ◦ energy changes in chemical reactions are determined by changes that occur in the chemical bonding ◦ Chemical reactions involve the breaking of chemical bonds in the reactants and the formation of chemical bonds in the products Chemical Bonds and Energy Breaking chemical bonds requires energy. ◦ Where could this energy come from when using a propane grill? Grills have a lighter which produces a spark, giving enough energy to break the bonds and start the reaction The formation of chemical bonds releases energy. (resulting in heat and light that you see) Breaking & Forming Bonds Physical changes can release or absorb energy ◦ Exothermic: releases ◦ Endothermic: absorbing ◦ During a chemical reaction energy is either released or absorbed ◦ A chemical reaction that releases energy to its surroundings is called an exothermic reaction ◦ A chemical reaction that absorbs energy from its surroundings is an endothermic reaction Endothermic and Exothermic Reactions •As you go from left to right in each graph, what happens to the reactants? •They react to form the products •What point on each graph represents the highest energy? •The energy is highest at each curve’s peak •What do the double-headed arrows represent? •The difference in chemical energy between the reactants and products •Which type of reaction has products with a greater amount of energy that the reactants? • endothermic Reaction rates 7.4 A reaction rate is the rate at which reactants change into products over time ◦ Rate just means a change over time, like distance over time= speed Reaction rates tell you how fast a reaction is going ◦ How fast reactants are consumed, products are formed or energy released/absorbed. Factors that affect reaction rates are: ◦ ◦ ◦ ◦ ◦ Temperature Surface Area Concentration Stirring Catalysts Reaction Rates How does temperature affect reaction rates? ◦ Increasing temp causes particles to move faster and collide, # of collisions increases then rate increases ◦ Decreasing the temperature will decrease the reaction rate Surface area is the amount of area exposed ◦ An increase in the surface area increases the exposure of reactants to one another allowing more collisions And therefore allowing an increase in the reaction rate (newspapers) Temperature and Surface Area Stirring increases the reaction rate by increasing the number of collisions between the particles of the reactants. ◦ (washing machine vs. soaking) Concentration is the number of particles in a given volume. (ex sugar in tea) ◦ The more particles of reactant, the higher the reaction rate Gas concentration changes with pressure (less room) ◦ The greater the pressure of the gas, the greater it’s concentration and the faster it’s reaction rate Stirring and Concentration A catalyst is a substance that affects the reaction rate without being used up in the reaction. They can be used to speed up or slow down reactions Graph shows how a catalyst can lower the amount of energy needed to cause a reaction Catalysts