Ex Credit Chem honors - Doral Academy Preparatory
advertisement

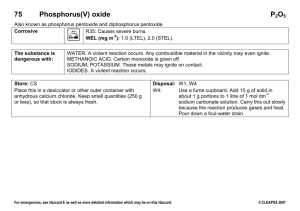
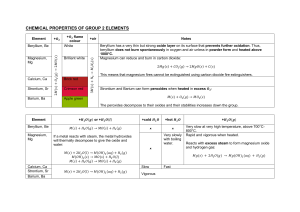
Name_______________________________________ Per______ Extra Credit Fill in the blanks. For the ions, include the charge. IONS FORMULA 1. Li+ and S2– 2. _____ and _____ 3. _____ and _____ 4. _____ and _____ 5. Fe3+ and Cl– 6. _____ and _____ 7. Al3+ and O2– 8. _____ and _____ 9. _____ and _____ 10. _____ and _____ NAME KHCO3 sodium bromide Mg(NO3)2 BaSO4 calcium carbonate NH4OH potassium phosphate Solve each problem. For full credit, show all work and include correct units, sig figs, and scientific notation if needed. 11. Three nails have a mass of 3.05 g. How many moles of iron do they contain? 12. A student needs to use 0.575 moles of sodium chloride in an experiment. How many grams should he mass out? 13. There are 17 key nutrients essential for bone health in the human body. The ideal intake of calcium is approximately 1.25 g per day; however, most adults only consume about 0.5 g per day in their diets. How many atoms of calcium does the average adult need daily? 14. Suppose that you massed out 3.50 g of Na2CO3. How many moles would you have? 15. What is the percent by mass of Cl in calcium chloride, CaCl2? 16. A 15.33 g sample of BaO is found to contain 13.73 g of barium. What is the percent by mass of the barium and oxygen in this compound? 17. A form of phosphorus called red phosphorus is used in match heads. When 0.062 g of red phosphorus burns in air, it forms 0.142 g of phosphorus oxide. Determine the empirical formula of phosphorus oxide. 18. Hydrazine can be used as a rocket fuel. A 6.4 g sample of hydrazine is found to contain 5.6 g of nitrogen and 0.80 g of hydrogen. The molar mass of hydrazine is the same as that of oxygen gas. Determine the empirical formula and the molecular formula of this compound. 19. Iron burns in air to form a black solid, iron (III) oxide. Write the balanced chemical equation. 20. Magnesium hydroxide reacts with nitric acid (HNO3) to form water and magnesium nitrate. Write the balanced chemical equation.