Crystal Violet Kinetics Lab
advertisement

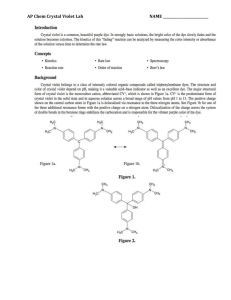
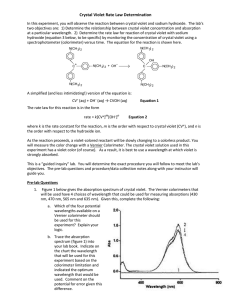
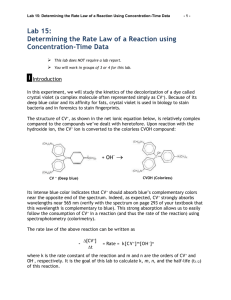
AP Chemistry Crystal Violet Kinetics Lab Crystal violet is an intense purple dye whose structure is pH dependent, which also makes it a possible acid-base indicator. When crystal violet is placed in a strong base the very noticeable violet colored solution will over time become colorless. A general equation for the reaction would look like: CV+ + OH- → CVOH Purple Colorless Writing a general rate law for this reaction would look like: Rate = k[CV+]x[OH-]y In this lab it is assumed that you will hold the concentration of hydroxide constant, so it could be included in the value of k, thus simplifying the rate law to look like: Rate = k[CV+]x In order to determine the order of the reaction with respect to crystal violet, you will find how the concentration of the crystal violet changes over time and graphically determine whether it is a zero, first, or second order reaction. The benefit of using crystal violet is that the concentration can be inferred by its absorbance of light. A solution of a known concentration of crystal violet in water will be used to determine an initial absorbance, and then the concentration of crystal violet at any given time can be calculated as proportional to the initial absorbance. Prelab Questions: (Use ink if not typed, and use complete sentences) 1. A light wavelength closest to 600nm will be used in this lab as crystal violet has its greatest absorbency near 600nm. Why is it good practice to use wavelengths corresponding to the greatest absorbency as opposed to choosing one with minimal absorbency? 2. In this lab 3mL of a solution of 0.000025M crystal violet will be mixed with 3mL of deionized water or 3mL of a 0.02M solution of sodium hydroxide. What will this make the final concentration of crystal violet? Show your work. 3. Explain thoroughly in words how the order of a reaction that is zero, first, or second can be determined graphically. Be sure to state the types of graphs needed and the key characteristics of the graphs that will indicate the order. Materials: 0.000025 M Crystal Violet Solution (0.5 mL of 1% solution to 500mL final volume) 0.02 M NaOH solution Pipet and pipet filler for 3mL 2 small test tubes Glove, goggles, and apron 3 cuvettes with lids Laptop with Loggerpro and colorimeter Test tube rack Procedures: 1. Set up the laptop with a colorimeter and start Logger Pro. 2. From the Experiments menu choose Data Collection. 3. For the mode choose Time Based. 4. Set the length to 900 seconds, and 30 seconds per sample. 5. Select Done. 6. Fill a cuvette with deionized water as your blank. 7. Place your blank into the colorimeter as be sure it reads very close to 100% transmittance at 565nm. If it does not read close to 100% push the calibration button (cal) on the colorimeter. If it still does not read close to 100% let Mr. Belland know so he can recalibrate the colorimeter. Then remove your blank. 8. In a test tube place exactly 3mL of crystal violet solution and 3mL of deionized water. With a gloved hand, shake the test tube well to mix. Pour this into a cuvette. 9. Place this cuvette into the colorimeter and record the absorbance. To find the absorbance right-click on the Transmittance box in the lower left corner and choose Digital Meter Options… 10. From the new box set the column to Absorbance, and choose OK. You should now see the absorbance of the crystal violet-deionized water mixture. Record this absorbance as abs0 (absorbance at time zero). Do not continue the lab until you have recorded this absorbance! 11. Remove the cuvette of crystal violet and deionized water. 12. In a test tube place exactly 3mL of crystal violet solution and 3mL of sodium hydroxide solution. With a gloved hand shake the test tube well to mix. Pour into a cuvette. 13. As quickly as possible insert the cuvette of crystal violet and sodium hydroxide into the colorimeter and click the Collect button. 14. While Logger Pro collects the data it would be a good time to clean up all the rest of the lab equipment. Crystal violet and sodium hydroxide may be washed down the drain with lots of water. 15. Logger Pro will automatically stop after 900s have elapsed. Double check that you have data in 30s intervals for 900s of absorbance. If that is what you see, then remove the final cuvette and clean it. 16. The analysis section depends on using Excel, or an Excel like program, so you need to export your data into a file that Excel can open. From the File menu choose Export As, and then CSV. 17. If you will not finish in class the information in this file needs to make it home with you, so save the file to your cloud, your U drive, a flashdrive, or print it out to type in at home. Without the contents of this file and the abs0 you recorded earlier you will not be able to continue. 18. Once the file is appropriately saved, you may continue on the laptop if time permits or if not you should shut down the laptop and return the equipment. Analysis: In Excel, or a program like Excel, open up the CSV file. You should have three columns with data: Time, Transmittance, and Absorbance. If you are typing in the data, you may leave out the Transmittance column. 1. 2. 3. Title a fourth column (column D) “Concentration”. This is the column for using the absorbance data to calculate the concentration of crystal violet at each time. Have Excel determine the concentration for you, by entering into the second box of the concentration column (column D) the formula “=0.0000125*C2/abs0” where you use the number you recorded for abs0 in place of that part of the equation. Then copy this to all the boxes below. Each time it should automatically change “C2” to “C3” to “C4”, etc. Title a fifth column (column E) “ln(concentration)”. This is the column for finding the natural log of the concentration values you just created in column D. Have Excel determine the natural log for you, by entering the formula “=ln(D2)” into the second box of the ln(concentration) column. Then copy this to all the boxes below. Again “D2” should change to “D3”, etc, as you copy. Title a sixth column (column F) “1/concentration”. This is the column for finding the reciprocal of the concentration values you created in column D. Have Excel determine the reciprocal for you, by entering the formula “=1/D2” into the second box of the 1/concentration column. Finally copy this to every box below. Your data and calculations table should look similar to this (but with your data): 4. Now prepare a graph of concentration vs time. In Excel hold down the Ctrl button and drag the curser over the concentration column (heading and data) and then over the time column (heading and data) and then with those two columns highlighted from the Insert Menu choose Scatter and Scatter with Smooth Lines. 5. A graph of Concentration vs Time should pop up. Move it to the side and continue. (If the graph does not look correct it is up to you to figure out how to make it – it is strongly suggested you check the internet for a how-to guide.) 6. 7. In a similar manner prepare graphs of ln(concentration) vs time and 1/concentration vs time. Arrange the three graphs around the data so that nothing is obstructed and it will all print on 1 or 2 pages. Once arranged, print and include the printouts in your lab report. Conclusion: (use ink if not typed, and use complete sentences) What is the order of this reaction (zero, first, or second)? Explain how you determined this. Postlab Questions: (use ink if not typed, and use complete sentences) 1. One of the key ideas of this lab was the assumption that the concentration of hydroxide ion remained constant, but as the crystal violet reacts it would remove some of the hydroxide ions from the solution. If it is assumed the reaction between crystal violet and hydroxide ion in the introduction went to completion, then calculate the percent error between the actual amount of hydroxide ion left after the reaction and the assumed constant value of 0.02M. It would be appropriate to show a RICE table as part of your work. Remember percent error is (assumedcalculated)/calculated. Finally state whether you feel this assumption is valid, citing the results of your error calculation. 2. Another key idea of this lab was the assumption that the order with respect to crystal violet was the overall order of the reaction. To actually determine the order of the reaction the order with respect to hydroxide ion would have to be determined. Why could we not determine the order of the reaction with respect to the hydroxide ion using colorimetry? What is one method that we might be able to use to track the change in concentration of hydroxide ion?