course objectives - Metropolitan Community College
advertisement

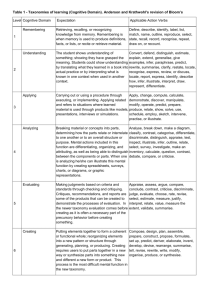
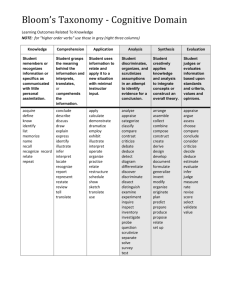
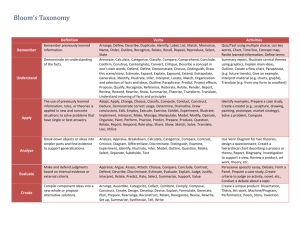
Metropolitan Community College COURSE OUTLINE FORM (Page 1 of 9) Course Title: Introduction to Physical Science Course Prefix & No.: LEC: LAB: SCIE 1010 5 3 Credit Hours: 6 COURSE DESCRIPTION: This is a survey course in the physical sciences with emphasis on scientific processes. It emphasizes the chemical and physical principles needed to better understand the world. It may also include topics from astronomy, geology and meteorology. COURSE PREREQUISITE (S): College-level reading, writing, and math proficiency; or assessment testing; and MATH 0931 or MATH 0960 RATIONALE: This course is a general survey for people who need a basic science course. The wide range of topics promotes an understanding of scientific methods and general knowledge of the physical world. REQUIRED TEXTBOOK (S) and/or MATERIALS: Title: An Introduction to Physical Science Edition: 2013/13 Author: Shipman, Wilson, and Todd Publisher: Cengage Learning Materials: Attached course outline written by: Patrick Nichols Date: SP/98 _ Reviewed/Revised by: Date: _ Effective quarter of course outline: 13/FA Date: _ Academic Dean: Date: _ Course Objectives, Topical Unit Outlines, and Unit Objectives must be attached to this form. ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 2 of 9) TITLE: Introduction to Physical Science PREFIX/NO: SCIE 1010 COURSE OBJECTIVES: Since it is not possible to cover all the areas of science included in this course in one quarter, the individual instructor may omit some sections. If the entire content is covered the student should be able to: 1. 2. 3. 4. 5. 6. Experiment logically using the scientific method. Explain the historical development of science. Describe the nature of matter. Explain basic mechanics, including: a. simple machines b. work c. power d. efficiency e. friction f. forces g. motion Explain the composition and systems of the earth. Develop some understanding of the Universe and solar system. TOPICAL UNIT OUTLINE/UNIT OBJECTIVES: I. A. B. DEVELOPMENT OF SCIENCE C. D. E. F. G. H. I. J. To be able to state many of the factors which influenced the rebirth of science in the 16th and 17th centuries. To be able to list the scientific contributions of early cultures, specifically those of the Chinese, the Babylonians, the Greeks, the Egyptians, and the Arabs. To be able to state the dominant goals of alchemy, the precursor of chemistry. To be able to explain the role of the Church of Rome, as it related to developing science in the 12th through 17th century. To be able to show how the development of gunpowder changed the political structure of the 14th century. To be able to tell why Galileo was called "the father of experimental science." To be able to list Galileo's major scientific works. To be able to list the steps in the scientific method. To be able to illustrate the basic difference between the inductive and deductive approaches to the understanding of nature. To be able to explain why Galileo's discovery of the phases of Venus disproved the Ptolemaic system. II. MEASUREMENT A. B. C. To be able to explain the historical background for the development of standardized units. To be able to distinguish between quantitative and qualitative approaches to communication. To be able to visually compare the following: 1. yard and meter 2. mile and kilometer 3. square foot and square meter 4. pound and newton To be able to convert miles to kilometers and vice-versa, readily, by approximation. To be able to explain the reasoning behind using water to define the kilogram. To be able to give a proper SI unit for each of the following: 1. time D. E. F. ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 3 of 9) G. H. III. A. B. C. D. E. F. G. H. I. J. IV. A. B. C. D. E. F. G. H. I. J. V. A. B. C. D. E. 2. length 3. area 4. volume 5. mass 6. density 7. weight To be able to state several advantages of the SI system and illustrate how it is being used, not only for science but also in everyday experience. To be able to clearly define the terms: 1. volume 2. mass 3. weight 4. density FORCE AND MOTION To be able to express newton's three laws of motion in your own words, as though you were explaining the concepts to a person for the first time. To be able to visualize velocities graphically - as a plot of distance versus time. To be able to distinguish between speed and velocity. To be able to define mass in terms of force and acceleration. To be able to use vectors to determine the heading necessary for an airplane to fly a certain course, given a wind heading and speed. To be able to resolve three forces into a single force, using vectors. To be able to explain how a rocket can be propelled (accelerated) to empty space. To be able to state several friction forces that are beneficial to our everyday experience and several that can be thought of as detrimental. To be able to give several illustrations of objects with vastly different momenta. To be able to give several illustrations of action-reaction in everyday life experiences. GRAVITY AND MOTION To be able to state all the factors which influence the force of gravity between two objects and tell how changes in any one of these factors affects that force. To be able to list at least ten examples in which friction is a retarding force and ultimately brings the object to rest when the motive force is removed. To be able to define and illustrate "action at a distance." To be able to explain why falling objects, of differing masses, experience the same acceleration due to gravity. To clearly distinguish between mass and weight. To be able to define centripetal and centrifugal force and specify the object on which each acts. To be able to express how the average velocity of a satellite is related to its average height above the earth. To be able to explain why planets orbit the sun, rather than moving in a straight line or literally falling into the sun. To be able to explain why objects of different masses fall side by side from a high tower (neglecting friction). To be able to illustrate Newton's Third Law (Action-Reaction) by an example from everyday experiences. ENERGY, WORK AND POWER To be able to illustrate the historical development of man's discovery and use of energy. To be able to list a number of ways in which you feel an overdependance upon energy today. To be able to suggest several new sources of energy, especially those which are non-polluting. To be able to illustrate the distinction between the common usage of the term "work" and its definition by physicists. To be able to define units such as: 1. newton 2. newton-meter 3. watt ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 4 of 9) F. G. H. I. J. VI. A. B. C. D. E. F. G. H. I. J. VII. A. B. C. D. E. F. G. H. I. J. VIII. A. B. C. D. E. F. G. 4. joule 5. horsepower and illustrate each in terms of everyday experience. To be able to demonstrate both the usefulness and limitations of simple machines. To be able to list several ways in which energy may be converted from one form to another. To be able to distinguish between temperature and heat, by giving accurate definitions of each. To be able to diagram temperature scales (F, C, K) and to know how to convert from one to another. To be able to illustrate the concept of entropy. WAVES AND SOUND To be able to draw the typical wave form of a displacement versus time graph of a vibrating object. To be able to illustrate the broad sense in which waves or wave forms describe natural phenomena. To be able to state the interrelationship between velocity, frequency and wavelength. To be able to distinguish between transverse and longitudinal waves. To be able to illustrate the degree to which sound influences our lives. To be able to state the difference between musical sounds and noise. To be able to draw an organ pipe which will produce a given note, assuming the wavelength of the note is known. To be able to calculate the harmonics of a given note. To be able to explain the characteristics of high fidelity music systems. To be able to define and illustrate a "standing Wave". ELECTRICITY AND MAGNETISM To be able to state the nature of the forces between like and unlike charges. To be able to diagram the reaction of an electroscope when a given charge is brought near or actually touched, showing concentrations of positive and negative charges in each case. To be able to write the mathematical relationship between force, charges and separation of two objects. To be able to express the difference between a static electric field and an electrical current. To be able to describe the process whereby a continuous electrical current may be created by a voltaic cell. To be able to work problems which involve voltage, current, and resistances. To be able to work problems involving both series and parallel resistors and compute the typical wattage of appliances which are used in a kitchen. To be able to state several different sources of magnetism. To be able to express the relationship between electricity and magnetism and state at least four applications which illustrate this relationship. To be able to graph the output of an alternating current generator in relation to the position of the armature. LIGHT To be able to list the specific classifications, within the electromagnetic spectrum, in the order of decreasing wavelength and tell how each is detected. To be able to state specifically what phenomena generates an electromagnetic disturbance. To be able to tell what happens when light: 1. travels in a vacuum 2. hits a mirror 3. passes into a glass of water 4. passes through a prism To be able to diagram the Bohr model of the hydrogen atom and show what king of electron transitions produce visible light. To be able to explain why the Doppler Effect occurs in a moving wave source and identify the effect in the spectrum of a star. To be able to relate the various parts of the human eye to those of a camera. To be able to draw light ray diagrams for the basic designs of reflecting telescopes: 1. prime focus 2. Newtonian ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 5 of 9) H. I. J. IX. A. To be able to explain why binoculars are so advantageous over the simple refracting telescope for terrestrial viewing - as in watching a football game. To be able to tell why a given atom (i.e. hydrogen) produces only certain spectral lines, not a full rainbow. To be able to contrast the microscope and the telescope in their basic purpose. NATURE OF ATOMS Q. R. S. T. To be able to clearly define and give examples of the terms: 1. atom 2. element 3. substance 4. mixture 5. molecule 6. compound To be able to relate the basic steps by which elements were found to ''fill" the periodic table, without leaving any gaps as far as the consecutive numbers of protons are concerned. To be able to explain how the periodic (repeating) nature of elements is evident from the definite proportions with which they combine and recite individual members of several chemical families. To be able to state the structural similarity of members of a given chemical family, at least in terms of their outer energy levels. To be able to diagram, using Lewis Dot Structures, all of the elements through Argon. To be able to state the properties which distinguish metals from non-metals. Given any atom with twenty or less protons, be able to determine the number of electrons in the principal energy level. To be able to explain ionic bonding and give several molecules which are formed by this kind of bonding. To be able to explain covalent bonding and give several molecules which are formed by this kind of bonding. To be able to define polar and non-polar bonds and give several everyday uses for each kind of molecule. To be able to trace the historical development of the model of the atom to the current model, recognizing the limitations in actually knowing the character. To be able to explain the concepts of definite proportions in the formation of molecules and give evidence for its validity. To be able to define radioactivity in atoms and picture the expulsion of alpha, beta, and gamma particles, along with their electrical natures. To be able to describe the physical and electrical characteristics of the electron and tell how its flow may be controlled. To be able to state the many practical uses of controlled electron flow in modern technology. To be able to define the word "quantum", in the context of modern physics, and state several evidences for the apparent validity of the notion. To be able to picture the particles within the nucleus of the atom and discuss the forces thought to hold them together. To be able to present the evidence which disproved the "raisin-pudding" model of the atom. To be able to relate the observations upon which Bohr built his model of the hydrogen atom. To be able to describe an experiment which gives evidence for the particle nature of light - the photoelectric effect. X. STATES OF MATTER A. B. C. To know the states of matter and to be able to distinguish between the nature of each state. To be able to state the kinetic theory of gas molecules and give evidence of their rapid motion. To know the mathematical relationship between volume, pressure, and temperature of a gas and to be able to work problems which ask for one unknown when the other facts are given. To be able to tell why pressure is directly proportional to temperature if volume is held constant. To be able to explain why liquids are virtually non-compressible and how this fact can be put to useful purposes. To be able to discuss the forces which tend to make a liquid "hang" together. To be able to draw the basic crystalline structures found in solids and tell why diamonds are so much harder than graphite, even though both are composed of carbon atoms. To be able to recite both the positive and negative aspects of the fact that water expands when it freezes. To be able to trace the energy requirements for heating 10 grams of ice from -10C, melting, raising the temperature to boiling and then boiling the water. To be able to define a plasma and tell where one typically exists. B. C. D. E. F. G. H. I. J. K. L. M. N. O. P. D. E. F. G. H. I. J. ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 6 of 9) XI. CHEMICAL CHARACTERISTICS A. B. To be able to define endothermic and exothermic reactions and give examples of each. To recognize chemical energy as potential energy within the bonds of molecules and to explain several sources of this kind of energy. To be able to define the terms oxidation and reduction and illustrate oxidation-reduction reactions. To demonstrate an ability to balance chemical equations. To be able to explain the significance of Avogadro's work in developing a relationship between the number of atoms (or molecules) and their atomic weight. To be able to compute the weight of Avogadro's number of molecules when given the chemical formula for that molecule, i.e. NaA102. To be able to define an acid and differentiate between strong and weak acids. To be able to define an acid and differentiate between strong and weak acids. To be able to define a base and differentiate between strong and weak bases. To be able to illustrate acid-base reactions. C. D. E. F. G. H. I. I. XII. BIOCHEMISTRY A. To appreciate the great variety of organic molecules that are possible through the bonding potential of the carbon atom and to be able to give at least ten examples of organic molecules. To be able to diagram a hydrocarbon chain when given the chemical formula, i.e. C6H12, and then construct several different isomers of the same molecule. To be able to recognize the subtle change which would convert a molecule like methane (CH4) into methyl alcohol (CH3OH). To be able to state the chemical equation for the process of photosynthesis. To be able to name at least three subcategories of carbohydrates. To be able to define a polyunsaturated fat. To be able to contrast soaps and detergents, including a discussion concerning their respective polluting qualities. To be able to explain the almost limitless nature of proteins. To be able to list at least ten sources of protein in the human diet. To be able to explain the role of DNA in the reproductive process. B. C. D. E. F. G. H. I. J. XIII. A. B. C. D. E. F. G. H. I. J. To be able to describe the fission process and identify its source of energy. To appreciate the problem in disposal of waste products. To be able to state the proton-proton cycle of fusion and identify its source of energy. To appreciate the difficulty in producing the conditions for fusion in a reactor. To be able to explain the role of the neutron in providing stability within an atom. To be able to explain the concept of the half-life of a radioactive element and tell how certain isotopes are used to date earthrocks, moon-rocks, and meteorites. To be able to tell why early chemists (called alchemists) were unable to turn lead into gold - one of their primary goals. To be able to relate some of the characteristics of sub-atomic particles that can be discovered by bubble chamber photos. To be able to state the source of energy in either the fission or the fusion process. To be able to state why the nucleus of an atom "hangs" together in spite of the fact that like charges repel each other. to be able to relate the source of fear in the fact that many countries are now capable of creating fission reactions. To be able to define the term 'critical mass", in relation to fissionable material. XIV. A. RADIOACTIVITY EARTH SCIENCE To be able to describe at least four ways in which the earth shows that it is still geologically active. ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 7 of 9) B. C. D. E. F. G. H. I. H. XIV. A. B. C. D. E. F. G. H. I. J. To be able to cite evidence for the location of an earthquake fault. To be able to describe volcanic activity and its source. To be able to give evidence for the fact that the earth's crustal plates move about. To be able to state at least six factors which cause erosion on the earth. To be able to relate the basic process which produces each type of rock: sedimentary, igneous, and metamorphic. To be able to define "radioactive element" and tell how such elements are used to find the age of rocks. To be able to list the principal geologic ages and give their approximate dates. To be able to state the basic assumptions geologists make. To be able to explain how oxygen (normally thought of as a gas) can be the major constituent of rocks. AIR AND WATER To be able to diagram the atmosphere by layers and give the temperature characteristic of each layer. To be able to discuss both the formation of the ozone layer and the threat to its destruction. To be able to describe the construction of a mercury barometer and explain its function. To be able to discuss the interrelationship between the ocean of air and the ocean of water. To be able to explain what factors about the earth and its motion produce the seasons. To be able to explain the factors about the earth and its motion which produce the prevailing winds and ocean currents. To be able to tell what causes tides on the earth, specifically explaining differential gravitational effects. To be able to list at least four of the principles upon which the science of weather prediction is based. to be able to diagram a hypothetical weather map, showing an advancing cold front. To be able to list at least four different air pollutants by chemical name and give their sources. XVI. MOON A. B. C. D. E. F. G. To be able to explain why the earth and moon can be called a binary system. To be able to explain a proof of the earth's rotation and give several consequences of that motion. To be able to explain a proof of the earth's revolution and give several consequences of that motion. To be able to describe the apparent motions of the usn, moon, and planets in relation to the signs of the zodiac in the sky. To be able to list the factors which produce precision in the earth and give at least two consequences of that motion. To be able to draw a diagram illustrating why the moon goes through phases. To be able to diagram the positions of the sun, moon, and earth to illustrate: 1. partial solar eclipse 2. total solar eclipse 3. total lunar eclipse To be able to explain how the tides are produced and why the moon is more effective in producing tides than the sun. To be able to tell what it would be like to live on the moon, in terms of: 1. surface features 2. temperature changes 3. appearance of the sky 4. erosion factors 5. etc. To be able to plot the moon each night for three weeks on a sky map. H. I. J. XVII. A. B. C. D. SOLAR SYSTEM To be able to recognize the planets which are visible during this course of study and state the constellation in which they are seen. To be able to explain why planets periodically appear to move westward among the stars, based on our current understanding of the solar system. (Their normal motion among the stars is eastward.) To be able to relate the major steps which brought us to an understanding that the sun is the center of the solar system. To be able to relate the major contributions of 1. Galileo 2. Brahe ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 8 of 9) 3. Kepler E. To be able to contrast the physical properties of the terrestrial and the Jovian planets and give several reasons why they are different. F. To be able to state at least two distinguishing characteristics of each planet. G. To be able to give several indications of the fact that Mars can be classified as geologically active. H. To be able to describe the astroid, the comet, and the meteor, including their true and apparent motions. I. To be able to contrast several theories for the origin of the solar system and relate the facts which seem to point to a single most favorable theory. J. To be able to generalize as to how scientists relate their theories to observed facts and what they expect from their theories. XVIII. A. B. C. D. E. F. G. H. I. J. K. L. M.. N. O. P. Q. COSMOS To be able to describe the physical nature of the sun as a model star. To be able to state the process whereby the sun (and stars in general) shines. To be able to explain how the scientist detects the composition of the sun (and stars) by observation of its spectra. To be able to define the range of star types with regard to size, temperature, and mass. To be able to explain why binary stars are useful in determining the masses of stars. To be able to characterize the life cycle of a star. To be able to state the physical properties of a black hole and explain how one may be detected. To be able to give examples of at least six different particles (and/or molecules) which are typically found int he space between the stars. To be able to discuss the role of radio astronomy in the research of interstellar molecules. To be able to list the different classifications of nebulae. To be able to rank in proper hierarchy the elements of the universe; namely, the following: 1. galaxies 2. clusters of galaxies 3. stars 4. moons 5. the sun 6. the planets To be able to sketch the basic shapes of the galaxies. To be able to relate the "Milky Way", as a fuzzy band of light in the sky, to the Milky Way, our galaxy. To be able to discuss the unusual galactic types, such as the quasar. To be able to cite evidence for the fact that the universe is expanding. To be able to present a coherent word picture of the origin and evolution of the universe. To be able to contrast an "open" universe with that which may be called "oscillating" (closed). ESO Revised 3-13-01 Metropolitan Community College COURSE OUTLINE FORM (Page 9 of 9) COURSE REQUIREMENTS/EVALUATION: Upon completion of the objectives of this course students will demonstrate thinking skills beyond memorizations of facts: to include comprehension, application, analysis, synthesis, and evaluation. COURSE OBJECTIVES/ASSESSMENT MEASURES COURSE OBJECTIVES ASSESSMENT MEASURES 1. 1. 2. 2. 3. 3. 4. 4. ESO Revised 3-13-01