starsinger51594.chemical reactions primer take 2
advertisement

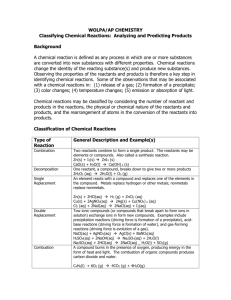
The part of atoms that are affected in chemical reactions are electrons. What’s happening?! Atoms of a same or different element are rearranging themselves to form a new substance through a chemical bond. Chemical Bond = Any of several forces or mechanisms, especially the ionic bond, covalent bond, and metallic bond, by which atoms or ions are bound in a molecule or crystal. Reactants = A substance participating in a chemical reaction, especially one present at the start. Product = A substance obtained from another substance through chemical change. Example = Na + Cl = NaCl In this formula the Na + Cl are the reactants and the NaCl is the product. In a chemical formula the reactants will always be on the left and the product will always be in the right. It states = States that mass is neither created nor destroyed in any ordinary chemical reaction. It relates because in a chemical reactions nothing is created or lost. what you end up with after the reaction may not look the same as when it started but there's the same amount off mass. Some may be gas while the rest is solids or liquids or whatever. Change in color Change Of Smell Or Taste Formation Of Precipitation More Signs Of A Chemical Reaction Change Of Temperature Change In Electrical Conductivity Bubbles Or Gas Appears Even More Signs Of A Chemical Reaction Light is emitted Change in volume A change in melting or boiling point Change in any distinctive chemical or physical property http://www.harpercollege.edu/tmps/chm/100/dgodambe/thedisk/chemrxn/signs.htm http://wiki.answers.com/Q/What_are_the_5_signs_of_a_chemical_re action The role of energy is that it is being exchanged. Chemical potential energy = a form of potential energy related to the structural arrangement of atoms or molecules. Activation energy = The least amount of energy needed for a chemical reaction to take place. When a chemical reaction is happening energy is released. For example, in an exothermic reaction the energy is being released in heat form. Exothermic = a chemical reaction that releases heat Endothermic = a chemical reaction that absorbs energy in the form of heat. melting ice cubes melting solid salts evaporating liquid water converting frost to water vapor splitting a gas molecule separating ion pairs cooking an egg baking bread reaction of barium hydroxide octahydrate crystals with dry ammonium chloride dissolving ammonium chloride in water reaction of thionyl chloride (SOCl2) with cobalt(II) sulfate heptahydrate mixing water and ammonium nitrate mixing water with potassium chloride reacting ethanoic acid with sodium carbonate photosynthesis What is it?! The forming or building of a more complex substance or compound from elements or simpler compounds. How can you tell?! In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. An example of synthesis sticking a lit wooden splint into a test tube of zinc and hydrochloric acid. For this lab we had to put some zinc metal into a test tube wit hydrochloric acid. Next we had to light a splint and stick it in the test tube producing a popping sound. What is it?! The breaking down of chemical reactants back into simple substances. How can you tell?! In a decomposition reaction a more complex substance breaks down into its more simple parts. One reactant yields 2 or more products. An example of decomposition is heating sodium bicarbonate and then sticking a lighted splint into the test tube with the sodium bicarbonate to see if there’s oxygen or carbon dioxide in the test tube. For this lab we had to collect a test tube and fill it with sodium bicarbonate. Then hold the test tube over a lit candle for about two minutes. Next we had to light a wooden splint and stick it in said test tube. What is it?! Rapid oxidation accompanied by heat and, usually, light. How can you tell?! Combustion reactions always involve molecular oxygen O2. One example of combustion is burning magnesium. For this lab we had to collect a candle(and light it) and a magnesium ribbon. We then had to pick up the magnesium ribbon with our tongs. Next we had to place the ribbon on the candles flame. What is it?! This reaction is when in a reaction a single uncombined element replaces a part of another compound forming into a different compound. How can you tell?! In a single replacement reaction a single uncombined element replaces another in a compound. Two reactants yield two products. An example of a single replacement is when we put aluminum foil into a beaker filled with CuCl2. For this lab we had to pour CuCl2 into a beaker. Then we put a piece of crumpled up aluminum foil into said beaker and watched it react. What is it?! A chemical reaction between two compounds where the positive ion of one compound is exchanged with the positive ion of another compound. How can you tell?! In a double replacement reaction parts of two compounds switch places to form two new compounds. Two reactants yield two products. An example of double replacement is when we mixed together sodium sulfate and barium chloride. For this lab we had to pour sodium sulfate into one test tube and then we had to pour barium chloride into another test tube. Next we mixed them together. What are the five signs of a chemical reaction? http://wiki.answers.com/Q/What_are_the_5_signs_of_a_chemical_reaction Signs Of A Chemical Reaction http://www.harpercollege.edu/tmps/chm/100/dgodambe/thedisk/chemrxn/signs.htm Exothermic Reactions http://www.enotes.com/forensic-science/exothermicreactions Endothermic http://en.wikipedia.org/wiki/Endothermic Endothermic Reaction Examples http://chemistry.about.com/od/lecturenotesl3/a/endorxns.htm What are some examples of endothermic and exothermic reactions? http://chemistry.about.com/od/lecturenotesl3/a/endorxns.htm Top Ten Amazing Chemical Reactions http://listverse.com/science/top-10amazing-chemical-reactions/ To React Or Not To React? That is the question! http://www.usoe.k12.ut.us/CURR/Science/sciber00/8th/matter/sciber/chemt ype.htm Chemical Synthesis http://www.usoe.k12.ut.us/CURR/Science/sciber00/8th/matter/sciber/chemt ype.htm Chemical Synthesis http://en.wikipedia.org/wiki/Chemical_synthesis Chemical Decomposition http://en.wikipedia.org/wiki/Chemical_decomposition Combustion http://en.wikipedia.org/wiki/Combustion Reaction Types: Single Replacement http://en.wikipedia.org/wiki/Combustion Reaction Types: Double Replacement http://dbhs.wvusd.k12.ca.us/webdocs/Equations/DoubleReplaceme nt.html What happens during a chemical reaction? http://www.enotes.com//science-experiments-projects/chemicalproperties/what-happens-during-chemical-reaction The Law Of Conservation Of Mass http://www.mi.mun.ca/users/edurnfor/1100/atomic%20structure/tsld 004.htm