biochem 9 [4-20
advertisement

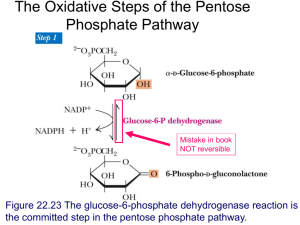
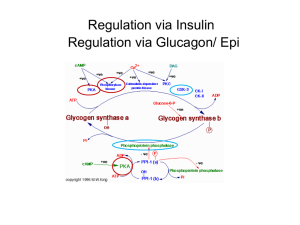
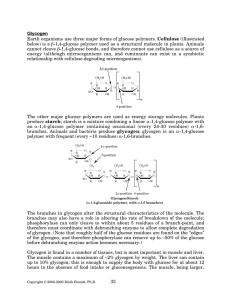
Biochemistry Chapter 9 Learning Objectives 1. How does a classic breathalizer work? Potassium dichromate (brown), sulfuric acid, and silver nitrate react with ethanol to produce potassium sulfate, chromium sulfate (green), acetic acid, and water Compare light absorption to nonreacted sample 2. What are the two routes for alcohol oxidation? ADH (Alcohol DeHydrogenase), which generates acetaldehyde and NADH MEOS (Microsomal Ethanol Oxidizing System), a member of the cytochrome P450 family 3. In the Michaelis-Menten Equation, what is the Km equal to? The Km of an enzyme is the substrate concentration ([S]) at which the reaction speed will be half of maximum (Vmax) What is a quick rule of thumb for the magnitude of Km? The larger the k of the forward reaction’s first part (Enzyme + S => ES), the smaller Km will be i. Km => 1/k1 4. 5. How does hexokinase differ from its isoenzyme glucokinase? Where are they found? Hexokinase is found in RBCs, and has an Km which is ~1/100th of glucokinase’s S0.5 which belongs in liver and pancreatic cells blood hex, pentagram of blood i. S0.5 is used for allosteric enzymes, which don’t exactly follow Michaelis-Menten equation RBCs can still phosphorylate glucose and make glycolysis happen at low [S] 6. If I double the amount of enzyme in my cells, what happens to the rates of production? Production will double as well! 7. In terms of Km, why does alcohol dehydrogenase (confusingly, often abbreviated ADH) exhibit 0-th order kinetics? ADH has an extremely small Km MEOS takes a greater part in ethanol oxidation at higher BACs 8. Why does alcoholism cause fatty liver change? NADH is a competitive inhibitor of ADH, which slows the rate of ethanol oxidation NADH is also a “product” inhibitor of enzymes in the pathway that oxidize fatty acids 9. What type of enzyme is generally composed of multiple subunits which show cooperativity in substrate binding? What do their plots of velocity versus substrate concentration look like? Allosteric enzymes Plots of velocity versus substrate concentration are S-shaped, rather than the rectangular hyperbolic shape of Michaelis-Menten kinetics 10. What is competition “in respect” to? Many competitive inhibitor block the place of just one of the substrates that an enzyme binds 11. What is the function of glycogen phosphorylase? What molecule is its activator? The enzyme breaks glycogen into glucose 1-phosphate Adenosine MonoPhosphate (AMP) functions as an allosteric activator of glycogen phosphorylase (it also activates phosphofructokinase-1, an enzyme involved in glycolysis) AMP increases when ATP concentrations drop, so that fuel oxidation increases Glycogen phosphorylase is phosphorylated when epinephrine levels rise, further activating it 12. What oversees the phosphorylation of glycogen phosphorylase? What stimuli (2) and enzymes (2) in turn control its activation? Glycogen phosphorylase kinase i. Phosphorylated by PKA or activated by calmodulin 1. Epinephrine=> Adenylate Cyclase=> cAMP=> PKA=> glycogen phosphorylase kinase=> => glycogen phosphorylase 2. nerves=> Ca++=> calmodulin=> glycogen phosphorylase kinase=> “ “ 13. What is the structure of inactive PKA? Two catalytic subunits are attached to two regulatory subunits The binding of cAMP dissociates the catalytic and regulatory subunits 14. What are GAPs, GEFs, and GDIs? GAPs increase the rate of GTP hydrolysis , inactivating a G-protein GEFs increase the rate of GTP Exchange for a bound GDP, activating a G-protein GDIs inhibit Dissociation of GDP, keeping the G-protein inactive If nothing else, E for excitatory, everything else inhibitory 15. What’s a zymogen? Give an example: Zymogens are the precursors of proteases, which cleave specific bonds as in digestion Trypsinogen is a zymogen, activated by enteropeptidase 16. What is a Lineweaver-Burk transformation? How does the difference between competitive and noncompetitive inhibition show up on such a graph? In competitive inhibition, plots of 1/v versus 1/[S] show an intersection of solutions containing inhibitor and normal solutions on the Y axis (ordinate); at ~0 [S], the reaction rate of a solution with inhibitor and substrate will be the same as one with only substrate In noncompetitive inhibition, they will intersect on the x axis (abscissa); the rate with inhibitor will always be a good deal slower