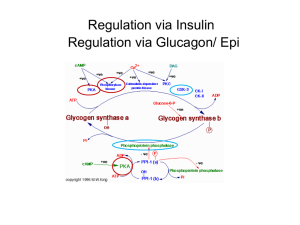
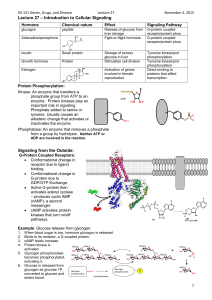
GLYCOGEN METABOLISM Dr. Phari Dajangju JNMC, AMU Introduction Storage form of Glucose Polymer held by glycosidic linkages Stored in Liver and Muscles Source of fuel for brain Structure In cytoplasm as granules inner linear chain Outer branched Central Glycogenin protein Function Storage form Energy for brain Reserve fuel for muscle contraction GLYCOGENOLYSIS Enzymes of Glycogenolysis Phosphorylase Glucose-6Phosphatase BifunctionalDebranching enzyme Phosphoglucomutase Step 1:Depolymerization (Release of Glu-1-P) Enzyme:Phosphorylase Co Enzyme:Pyridoxal phosphate Orthophosphate splits between C1 and C4 of adjacent glucose Stops 4 units before branching point Step 2: Remodelling & Debranching Bifunctional enzyme Transferase Alpha-1,6-glucosidase Release of free glucose residue Linear Glycogen Step 3:Conversion of Glu-1-P to Glu-6-P Enzyme: Phosphoglucomutase The active site of Mutase has phosphorylated serine Glu-1,6-bisP intermediate formed Step 4: Fate of Glu-6-P Reaction catalysed by Phosphotase Regulation of Glycogenolysis Regulation of enzyme Glycogen Phosphorylase Hormonal action of Glucagon/Epinephrine Mechanism is similar in Liver and Muscle Epinephrine in Muscle Glycogen Phosphorylase 2 Isozyme Glycogen Phosphorylase a and b Each exists in R and T state Dimer with Serine residue Phosphorylase kinase promotes Phosphorylase b—>a Phosphorylase kinase Structure4 subunits, 4 of each Activation1.Phosphorylation of beta -partial activation 2.Calcium attaches to delta-full activation Regulation of glucose breakdown in muscles Low energy state1.Epinephrine through Phosphorylase kinase Phosphorylase b—->a 2.AMP from degradation of ATP as allosteric activator of Phosphorylase b from T—>R state Resting state1.ATP shift Phophorylase b R—>T state 2.High Glu-6-P favour T state Regulation of glycogen breakdown in Liver Liver Phosphorylase sensitive to free Glu Free Glu binds to active site, causes covalent modification R—>T state Low Glu..activate Phosphorylase a Insulin promotes uptake of Glu and phosphorylation to Glu-6-P Glucagon signal pathway Alpha cells of pancreas secrete Glucagon Epinephrine by adrenal medulla Epinephrine bind to alpha adrenergic receptor Similar cascade of reactions Role of Insulin in Glycogen degradation Both Phosphorylase and Phosphorylase kinase are dephosphorylated and inactivated by protein phosphatase. Protein phosphatase is stimulated by Insulin, Therefore Insulin by inhibiting the activation of these enzymes inhibits the overall process of glycogenolysis. 14-Jan-17 NAMRATA CHHABRA, M.D. GLYCOGENESIS GLYCOGENESIS Mainly in Liver and Muscles Liver Glycogen functions as storage and export of glucose for maintaining level Muscle glycogen as readily available source of glucose Phases of Glycogenesis Activation Initiation Glycogen Branching Elongation ACTIVATION OF GLUCOSE Step 1:Phosphorylation of Glucose Step 2:Conversion of Gluc-6-P to Gluc-1-P Step-3- Conversion of Glucose-1-P to UDP-Glucose Enzyme UDP Glu Pyrophosphorylase INITIATION Initiation Glycosidic bond between the C1 of the glucose moiety of UDPglucose and the hydroxyl oxygen of a tyrosine side-chain of Glycogenin. Glucosyl Transferase UDP is released as a product. Each subunit of glycogenin catalyzes the addition of eight glucose units to its partner in the glycogenin dimer. At this point, glycogen synthase takes over to extend the glycogen molecule. Elongation Catalyzed by Glycogen Synthase New glucosyl units added to nonreducing terminal residues of glycogen. Formation of α-1,4-glycosidic linkage. . Incorporation of UDP-Glucose intoglycogen Glycogen Branching Amylo-(1,4)—>(1,6)-transglucosidase Increases the solubility Increases terminal residue which are sites for action of Synthase and Phosphorylase Regulation of glycogen synthesis Glycogen Synthase GS 1 in muscles, GS2 in Liver Phosphorylation inactivates a-->b Glu-6-P is allosteric effector Role of Insulin in Glycogenesis Promotes Glycogenesis Causes activation of Phosphoprotein Phosphatase resulting dephosphorylation of Glycogen Synthase In liver insulin increases the activity of phosphodiesterase, promoting hydrolysis of cAMP Insulin thus antagonizes effects of the cAMP cascade induced by glucagon & epinephrine. 14-Jan-17 NAMRATA CHHABRA, M.D. REGULATION General mechanisms involved in the regulation of enzyme activities Regulation of enzyme activity Induction/Repres sion Covalent modification 14-Jan-17 Allosteric modification NAMRATA CHHABRA, M.D. Substrate/produ ct concentration Key enzymes involved in the regulation of glycogen metabolism Glycogen synthaseFor Glycogenesis Glycogen Phosphorylase NAMRATA CHHABRA, M.D. Both these enzymes are reciprocally regulated. Reciprocal regulation of Enzymes Glycogen Synthase & Phosphorylase activity are reciprocally regulated At the same time as phosphorylase is activated by a rise in concentration of cAMP (via phosphorylase kinase), glycogen synthase is converted to the inactive form. Thus, inhibition of glycogenolysis enhances net glycogenesis, and inhibition of glycogenesis enhances net glycogenolysis Both processes do not occur at the same time. 14-Jan-17 NAMRATA CHHABRA, M.D. Substrate concentration and allosteric modification Substrate Glucose-6-P Glycogen Synthase is allosterically activated by glucose-6-P. High blood glucose concentration leads to elevated intracellular glucose-6-P. When glycolytic pathway is saturated, excess glucose-6-P activates Glycogen synthase 14-Jan-17 NAMRATA CHHABRA, M.D. Covalent modification- General concepts Reversible phosphorylation and dephosphorylation Hormone mediated cAMP mediated cascade Phosphorylation is mediated by Protein kinase A Dephosphorylation is carried out by Phosphatase Insulin causes dephosphorylation by stimulating Phosphatase and Phosphodiesterase (enzyme that breaks down cAMP) NAMRATA CHHABRA, M.D. Glucagon14-Jan-17 causes phosphorylation by stimulating Protein kinase A Regulation of glycogen synthase by covalent modification Glycogen synthase exists in both phosphorylated or dephosphorylated states Active glycogen synthase a is dephosphorylated and inactive glycogen synthase b is phosphorylated 14-Jan-17 NAMRATA CHHABRA, M.D. Covalent modification of glycogen synthase Glycogen synthase a Pi Phosphatase H2O 14-Jan-17 Active p Glycogen synthase b Inactive NAMRATA CHHABRA, M.D. ATP Protein kinase A ADP Regulation of Glycogenolysis by Covalent Modification The cAMP cascade results in phosphorylation of a serine hydroxyl of Glycogen Phosphorylase, which promotes transition to the active state. The phosphorylated enzyme is less sensitive to allosteric inhibitors. Thus, even if cellular ATP and glucose-6-phosphate are high, Phosphorylase will be active. 14-Jan-17 NAMRATA CHHABRA, M.D. Role of cAMP In Glycogen degradation cAMP activates cAMP dependent Protein Kinase Phosphorylation of inactive phosphorylase kinase b to a Phosphorylation of inactive Glycogen Phosphorylase b to a In the liver, cAMP is formed in response to glucagon, muscle is insensitive to glucagon. In muscle, increased cAMP formation is the action of norepinephrine 14-Jan-17 NAMRATA CHHABRA, M.D. Role of calcium in muscle degradation Phosphorylase Kinase is partly activated by binding of Ca++ Phosphorylase Kinase Dephosphorylated (inactive) Ca++ Further activation is brought by phosphorylation. 14-Jan-17 NAMRATA CHHABRA, M.D. Phosphorylase kinase- Ca++ Partly active ATP Phosphorylase kinase- Ca++ Phosphorylated- active THANK YOU