Periodic Trends
advertisement

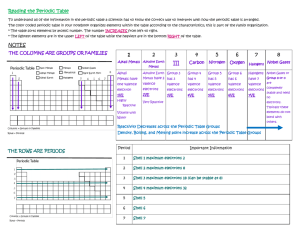
Organization of the Periodic Table PERIODS Columns of the periodic table Atoms of elements in the same group have the same # of valence electrons and therefore behave similarly •Rows of the periodic table •All elements in a period have their valence electrons in the same energy level. 1. 2. 3. 4. H, He, C, Li K, Ca, As, Br He, Ne, Kr, Ar B, Al, Ge, Sn 0% 0% 0% 0% 1. 2. 3. 4. H, He, C, Li K, Ca, As, Br He, Ne, Kr, Ar B, Al, Ge, Sn 0% 0% 0% 0% Valence Electrons are electrons in the outermost energy level. - s or p electrons only (even when d and f electrons are present they are not in the outermost energy level) Electron Dot Diagrams show the valence electrons of an element. Draw the electron dot diagrams for the following: Mg N F 1. 2. 3. 4. 1 2 7 8 0% 0% 0% 0% 1. 2. 3. 4. 1 2 7 8 0% 0% 0% 0% 1. 2. 3. 4. 5. 1 2 7 8 Unable to tell 0% 0% 0% 0% 0% Think of a ball of an onion. What happens with each layer? As you go down the periodic table, the energy levels increase and the size of the radius of the atom increases. (each energy level is like another layer of the onion) 1. 2. 3. Na K Unable to be determined 0% 0% 0% 1. 2. 3. Br Cl Unable to be determined 0% 0% 0% • • • As you move from left to right in the periodic table, what happens to the number of protons in the nucleus? What effect do these protons have on the electrons? What effect do the electrons have on each other? • Electron Shielding (or Screening) – These inner electrons shield the valence electrons from receiving the entire attractive nuclear charge because they repel the valence electrons. Within a period, as you go from left to right, the positive nuclear charge increases, and attracts the electrons more strongly. As the electrons are more attracted to the nucleus, the atomic radius decreases. Summary: as you go from left to right, the atomic radius generally decreases. 1. 2. 3. Li Be Unable to be determined 0% 0% 0% 1. 2. 3. Si Ar Unable to be determined 0% 0% 0% 1. 2. 3. Be Mg Unable to be determined 0% 0% 0% 1. 2. 3. C Si Unable to be determined 0% 0% 0% The octet rule states that all atoms attempt to become stable by having a full valence electron shell (generally 8 electrons, hence octet rule). Atoms will gain, lose, or share electrons in order to attain this stability. 1. 2. 3. 4. Alkali metals Transition metals Halogens Noble Gases 0% 0% 0% 0% 1. 2. 3. 4. 5. 6. 7. Alkali metals Transition metals Halogens Alkaline earth metals Both alkali metals and halogens Noble gases Both alkali metals and alkaline earth metals 14% 14% 14% 14% 14% 14% 14% Electrons are held in atoms by their attraction to the positively charged nucleus. To remove an electron requires energy. Ionization energy is the energy required to remove the least tightly bound (or outermost) electron from an atom. Compare Li and K. How many valence electrons? What is the relative size of the atoms? Which has a higher ionization energy? As you go down a group, the ionization energy decreases because it takes less energy to remove an electron. The least tightly bound electrons are further from the positive nucleus, and can therefore be removed more easily. 1. 2. 3. 4. Halogens Alkali Metals Alkaline Earth Metals All the same 0% 0% 0% 0% 1. 2. 3. 4. Li Be F Ne 0% 0% 0% 0% 1. 2. 3. 4. Na Mg S Ar 0% 0% 0% 0% Ionization energy generally decreases as you go down a group and from right to left in a period. 1. 2. 3. 4. 5. 1 3 4 6 8 0% 0% 0% 0% 0% 1. 2. 3. 4. 5. 6. 7. K Na Li Be Cs Ca F 0% 0% 0% 0% 0% 0% 0% 1. 2. 3. 4. 1 2 3 None of the above 0% 0% 0% 0% 1. 2. 3. 4. 5. 6. 7. K Na Li Be Cs Ca F 0% 0% 0% 0% 0% 0% 0% 1. 2. 3. 4. 1 4 7 14 0% 0% 0% 0% Second ionization is the energy required to remove a second electron. Ex. Sodium has a lower (first) ionization energy than Magnesium but Mg has a lower second ionization energy than Na. Why? 1. 2. 3. 4. K Ca Ga C 0% 0% 0% 0% Electronegativity is the tendency of an element to attract electrons in a bond. Therefore, elements that want to gain electrons will have higher electronegativity. attractive 1. 2. 3. 4. Alkali Metals Transition Metals Halogens Noble Gases 0% 0% 0% 0% 1. 2. 3. 4. 5. 6. 7. K Na Li Be Cs Ca F 0% 0% 0% 0% 0% 0% 0% 1. 2. 3. 4. Alkali metals Alkaline earth metals Halogens Transition Metals 0% 0% 0% 0% The pattern for increasing electronegativity (except for noble gases). The pattern for increasing ionization energy. The pattern for increasing atomic radius. When an atom loses or gains electrons, it gains a charge. An ion is a charged atom. A positive ion is called a cation. (ex. Na+) A negative ion is called an anion. (ex. S2-) Cations (positive) – lose valence electrons in the outermost energy level. ◦ They lose an energy level so they get smaller. Anions (negative) – gain valence electrons but their Zeff does not change. ◦ They get bigger because of having more electrons and the same Zeff 1. 2. 3. Mg Mg+ Mg2+ 0% 0% 0% 1. 2. 3. O OO2- 0% 0% 0% 1. 2. 3. Ca Ca+ Ca2+ 0% 0% 0% 1. 2. 3. S SS2- 0% 0% 0% Groups 1-13 become cations by losing electrons because of their low ionization energy. Their positive charge corresponds to their group number. Groups 15-17 become anions by gaining electrons because of their high electronegativity. Their negative charge corresponds to how many electrons they must gain to have the same electron configuration as a noble gas. 1. 2. 3. 4. Ionization energy Atomic radius Thermal capacity Electronegativity 0% 0% 0% 0% 1. 2. 3. 4. Aluminum Oxygen Magnesium Lithium 0% 0% 0% 0% 1. 2. 3. 4. Atomic radius Ionization Energy Atomic Mass Ionic Charge 0% 0% 0% 0% 4 Different kinds of metals ◦ Alkali metals: soft, shiny and very reactive Group 1: not found in nature as elements ◦ Alkaline earth-metals: less reactive Group 2: have two valence electrons ◦ Transition Metals: many uses Groups 3-12 3 Different kinds of metals ◦ Noble Gases: mostly non-reactive, very stable Group 8: He, Ne, Ar, Kr, Xe, Rn ◦ Halogens: very reactive, gain one electron to form a stable compound Group 7: F, Cl, Br, I Seven elements are called diatomic and never exist alone in nature. Have No Fear Of Ice Cold Beans????? H2 N2 F2 O2 I2 Cl2 Br2 1. 2. 3. 4. 5. Alkali metals Alkaline earth metals Transition metals Halogens Noble gases 0% 0% 0% 0% 0% 1. 2. 3. 4. 5. Alkali metals Alkaline earth metals Transition metals Halogens Noble gases 0% 0% 0% 0% 0% 1. 2. 3. 4. 5. Alkali metals Alkaline earth metals Transition metals Halogens Noble gases 0% 0% 0% 0% 0% 1. 2. 3. 4. 5. Alkali metals Alkaline earth metals Transition metals Halogens Noble gases 0% 0% 0% 0% 0% 1. 2. 3. 4. 5. Alkali metals Alkaline earth metals Transition metals Halogens Noble gases 0% 0% 0% 0% 0% Metals ◦ Shiny ◦ Solids ◦ Stretched and Shaped ◦ Conductors of heat and electricity Nonmetals ◦ Solids, liquids or gases ◦ Solids – dull and brittle ◦ Poor conductors of heat and electricity ****Semiconductors / Metalloids – exhibit properties of both metals and nonmetals Metals ◦ On the left hand side of the zigzag line (except for Hydrogen – exception) - Metalloids or Semimetals - Touching zigzag line (Except for Al) - Exhibit properties of both metals and nonmetals Nonmetals ◦ On the right hand side of the zig zag line (plus Hydrogen) The difference in electronegativity between atoms A and B is given by: