CHEMICAL BONDING
advertisement
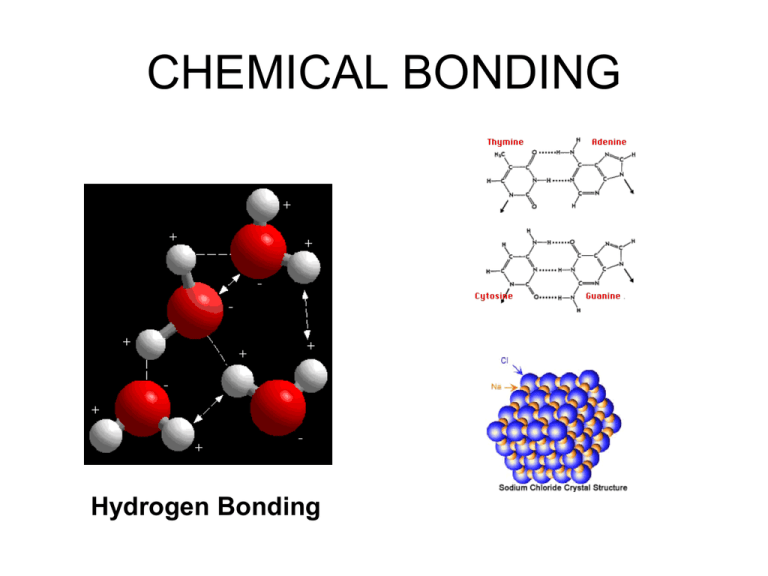
CHEMICAL BONDING Hydrogen Bonding Chemical Bonding Unit Skills • Compare ionic, covalent, and metallic bonds • Draw molecular structures • Distinguish between polar and nonpolar compounds • Explain the affect of hydrogen bonds on compounds and how the are formed This unit corresponds with chapter 12 Chemical Bonds • Force of attraction between two atoms • Several kinds of bonds: – Ionic bonds • Network solids – Covalent bonds • Macromolecules • Covalent I & II – Metallic bonds + metal Ionic Bonds nonmetal • Occur in ionic compounds generally between a metal and a nonmetal • Formed when electrons are transferred • Strong bond • High melting point • Generally solids • Poor conductors of electricity as a solid, but good conductors when dissolved Covalent Bonds • Generally occur in molecular compounds between two nonmetals • Formed when electrons are shared • Weak bonds *** • Low melting points • Usually liquids or gases • Poor conductors of electricity Polar covalent bonding Occurs when 2 slightly different atoms share electrons unequally to be more stable. The electrons are not completely transferred but an unequal sharing results. We use these symbols to show which atom has a stronger attraction for the electrons. Which of the following compounds contains ionic bonds? 1. 2. 3. 4. NaCl NO F2 SO3 Sharing in covalent bonds • Electronegativity determines how electrons are shared • Uneven sharing = polar bond • Even sharing = nonpolar bond ••• • Electronegativity What element the hasattraction, the highest Theelements stronger theelectronegativity? higher the number. The have been assigned numbers according to their What the lowest? ability has to “attract” electrons to itself from the shared bond. A bond’s polarity depends on the difference in the electronegativity values of the atoms in the bond. HF 2.1 4.0 H Cl 2.1 3.0 4.0 The HF -2.1 bond is 1.9 3.0 -2.1 0.9 more polar Metallic Bonds • Occur between atoms of a metal of the same element. • Strong bonds • Have all of the properties of metals discussed with the periodic table Metals form a regular array of atoms. But the valence electrons form a “sea” of electrons also called “delocalized”. Intermolecular Attractions Why is carbon a solid at room temperature and water a liquid? Dipoledipole attractions are 1% as strong as regular bonds. Different attractions between molecules determine the properties and states of matter. Hydrogen Bonds • Bond that occurs between molecules containing hydrogen and an atom with a high electronegativity (usually N, O, F, Cl, or S) Hydrogen bonds are strong intermolecular attractions… about 10 x stronger than dipole-dipole attractions London dispersion forces are another Even non-polar matter. molecules can form weak attractions. This is due to the uneven distributions of electrons at any one time. Lewis Structures (Dot Diagrams) • Used to show the arrangement of atoms in molecules or ions • Follow the steps to draw Lewis structures • But first a quick review Let’s try bromine. Notice: bromine now has 8 electrons! 2 10 5 4s 3d 4p Br [Ar] 2 10 6 Br [Ar] 4s 3d 4p How many valence electrons? What would the bromine ion look like? H2 H H By sharing electrons, each hydrogen has, in effect, 2 electrons or a filled valence shell (stable duet) Why doesn’t Helium form bonds? He How about F2? F F Each fluorine has 7 valence electrons. That is 1 bonding pair and 3 “lone pairs” or “unshared pairs” Does Neon form bonds? Ne Now let’s look at families on the periodic table. Li Na K Rb Cs Fr O [He] 2s1 S [Ne] 3s1 [Ar] 4s1 [Kr] 5s1 Se [Xe] 6s1 [Rn] 7s1 Te H O H H - O- H Count the total valence electrons: 1 + 6 + 1 = 8 Place the electrons to form a bond between atoms and to complete the duet and octet. Often, instead of showing 2 dots between atoms for a bond, we draw a bar. Sometimes we have multiple bonds! CO2 O=C=O O-C-O Count the total valence electrons:6 + 4 + 6 = 16 The counts work but both oxygen atoms do not have a stable octet. This is the best arrangement for CO2, but is there another? O=C=O O-C=O O=C−O These are called resonance structures. A molecule shows resonance when more than one Lewis structure can be drawn. We will discuss later which one is the most stable. Try another: CN C-N Count the total valence electrons:4 + 5 + 1= 10 Are there any resonance structures? Try these: HF •• • H-F • •• N2 •• N = N•• •• NH3 CH4 H–N–H H H H–C–H H | | F | F – C – F| 4 | F| CF + NO •• N - = O•• -NO3| O – N = O| - | | O - More to practice on your own! a. NF3 b. O2 c. CO d. PH3 e. H2S f. SO42g. NH4+ h. ClO3i. SO2 Steps for drawing Lewis structures 1. Count the number of valence electrons in the molecule or ion 2. Arrange the atoms around a central atom 3. Put a pair of electrons (2 dots) where each bond occurs 4. Put the remaining electrons around each atom so all have 8 except hydrogen which can only have 2 Some exceptions to the octet rule • A few atoms seem to be stable being “electron deficient” • The atoms are Be, B, Mg and Al • So we can have molecules like BF3 F I’ve left off the electrons around the fluorine atoms due to time & space. F B F VSEPR Model • • • • • Valence Shell Electron Pair Repulsion When bonds form, several forces come in to play… The bonding electrons let atoms achieve a noble gas configuration (lowering energy). A small concentration of negative charge forms between the two atoms. Also, there may be unbonded pairs of electrons. These create a very large concentration of negative charge and these repel other areas of negative charge. Basic structures & predicting • • • • • • • • • • • Linear Trigonal planar Bent & v-shape Tetrahedral Trigonal pyramidal Trigonal bipyramidal See saw T-shape Octahedral Square pyramidal Square planar We’ll take these one at a time. Notation AXE • A represents the central atom • X represents the ligands (atoms bonded to central atom) • E represents the unbonded electron pairs Linear Cl Be Cl Bond angle 180o BeCl2 Both electron Hybridization: and 2 0 sp molecular geometry are central atom ligands linear. unbonded electron pairs AX E Trigonal planar F F B F Both electron and molecular geometry are trigonal planar. A X3 E0 Bond angle 120o BF3 Hybridization: sp2 central atom ligands unbonded electron pairs Bent O S Electron geometry is trigonal planar and molecular geometry is bent or v-shape O Bond angle less than 120o SO2 A X2 E1 central atom ligands unbonded electron pairs Hybridization: sp2 Tetrahedral H H CH H Both electron and molecular geometry is tetrahedral. Bond angle 109.5o A X4 E0 CH4 Hybridization: sp3 Trigonal Pyramidal H NH H Electron geometry is tetrahedral and molecular geometry is trigonal pyramidal. Bond angle 107o A X3 E1 NH3 Hybridization: sp3 Very Bent H O H Electron geometry is tetrahedral and molecular geometry is bent or very bent. Bond angle 104.5o AX2 E2 H2O Hybridization: sp3 Trigonal bipyramidal Leaving off the unshared electron pairs on F due to space. F F F P F F Both the electron and molecular geometry is trigonal bipyramidal PF5 Bond angles 90o and 120o AX5 E0 Hybridization: sp3d See saw Leaving off the unshared electron pairs on F due to space. F F S F F Electron geometry is trigonal bipyramidal, molecular geometry is see saw or distorted tetrahedral SF4 Bond angles 90o and 120o AX4 E1 Hybridization: sp3d T-shaped Leaving off the unshared electron pairs on F due to space. F F ClF3 Bond angles 90o Cl F Electron geometry is trigonal bipyramidal, molecular geometry is T-shaped AX3 E2 Hybridization: sp3d Linear Leaving off the unshared electron pairs on F due to space. F XeF2 Bond angles 180o Xe F Electron geometry is trigonal bipyramidal, molecular geometry is linear AX2 E3 Hybridization: sp3d Octahedral Leaving off the unshared electron pairs on F due to space. F F F S F F F SF6 Bond angles 90o Both electron geometry and molecular geometry is octahedral AX6 E0 Hybridization: sp3d2 Square Pyramidal Leaving off the unshared electron pairs on F due to space. F F F Br F F BrF5 Bond angles 90o Electron geometry is octahedral, molecular geometry is square pyramidal AX5 E1 Hybridization: sp3d2 Square Planar Leaving off the unshared electron pairs on F due to space. F F Xe F F XeF4 Bond angles 90o Electron geometry is octahedral, molecular geometry is square planar AX4 E2 Hybridization: sp3d2 Try these: What is the molecular geometry of GeI4? 4 + 4(7) = 32 I Tetrahedral I Ge I I AX4 E0 Try these: What is the molecular geometry of GeI4? 4 + 4(7) = 32 I Tetrahedral I Ge I I AX4 E0 Molecular Structure Worksheet Molecules • • • • • • • • • Carbon tetrachloride Phosphorus trichloride Hydrogen sulfide Chlorine molecule (Cl2) Sulfur dichloride Carbon monoxide Ethane (C2H6) Silicon dioxide Hydrogen cyanide Molecular Shapes What happens when oil is put in water? • Like Dissolves Like Cell Membranes